Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus
- PMID: 26246580
- PMCID: PMC4580173
- DOI: 10.1128/JVI.00304-15
Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus
Abstract
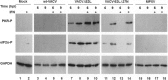
The vaccinia virus (VACV) E3 protein has been shown to be important for blocking activation of the cellular innate immune system and allowing viral replication to occur unhindered. Mutation or deletion of E3L severely affects viral host range and pathogenesis. While the monkeypox virus (MPXV) genome encodes a homologue of the VACV E3 protein, encoded by the F3L gene, the MPXV gene is predicted to encode a protein with a truncation of 37 N-terminal amino acids. VACV with a genome encoding a similarly truncated E3L protein (VACV-E3LΔ37N) has been shown to be attenuated in mouse models, and infection with VACV-E3LΔ37N has been shown to lead to activation of the host antiviral protein kinase R pathway. In this report, we present data demonstrating that, despite containing a truncated E3 homologue, MPXV phenotypically resembles a wild-type (wt) VACV rather than VACV-E3LΔ37N. Thus, MPXV appears to contain a gene or genes that can suppress the phenotypes associated with an N-terminal truncation in E3. The suppression maps to sequences outside F3L, suggesting that the suppression is extragenic in nature. Thus, MPXV appears to have evolved mechanisms to minimize the effects of partial inactivation of its E3 homologue.
Importance: Poxviruses have evolved to have many mechanisms to evade host antiviral innate immunity; these mechanisms may allow these viruses to cause disease. Within the family of poxviruses, variola virus (which causes smallpox) is the most pathogenic, while monkeypox virus is intermediate in pathogenicity between vaccinia virus and variola virus. Understanding the mechanisms of monkeypox virus innate immune evasion will help us to understand the evolution of poxvirus innate immune evasion capabilities, providing a better understanding of how poxviruses cause disease.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, Schriewer J, Buck C, Wang C, Lefkowitz EJ, Esposito JJ, Harms T, Damon IK, Roper RL, Upton C, Buller RM. 2005. Virulence differences between monkeypox virus isolates from West Africa and the Congo Basin. Virology 340:46–63. doi:10.1016/j.virol.2005.05.030. - DOI - PMC - PubMed
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication, vol 6 World Health Organization, Geneva, Switzerland.
-
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. 2004. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med 350:342–350. doi:10.1056/NEJMoa032299. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

