Gene Therapy Fully Restores Vision to the All-Cone Nrl(-/-) Gucy2e(-/-) Mouse Model of Leber Congenital Amaurosis-1
- PMID: 26247368
- PMCID: PMC4575531
- DOI: 10.1089/hum.2015.053
Gene Therapy Fully Restores Vision to the All-Cone Nrl(-/-) Gucy2e(-/-) Mouse Model of Leber Congenital Amaurosis-1
Abstract
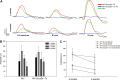
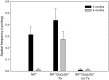
Mutations in GUCY2D are the cause of Leber congenital amaurosis type 1 (LCA1). GUCY2D encodes retinal guanylate cyclase-1 (retGC1), a protein expressed exclusively in outer segments of photoreceptors and essential for timely recovery from photoexcitation. Recent clinical data show that, despite a high degree of visual disturbance stemming from a loss of cone function, LCA1 patients retain normal photoreceptor architecture, except for foveal cone outer segment abnormalities and, in some patients, foveal cone loss. These results point to the cone-rich central retina as a target for GUCY2D replacement. LCA1 gene replacement studies thus far have been conducted in rod-dominant models (mouse) or with vectors and organisms lacking clinical translatability. Here we investigate gene replacement in the Nrl(-/-) Gucy2e(-/-) mouse, an all-cone model deficient in retGC1. We show that AAV-retGC1 treatment fully restores cone function, cone-mediated visual behavior, and guanylate cyclase activity, and preserves cones in treated Nrl(-/-) Gucy2e(-/-) mice over the long-term. A novel finding was that retinal function could be restored to levels above that in Nrl(-/-) controls, contrasting results in other models of retGC1 deficiency. We attribute this to increased cyclase activity in treated Nrl(-/-) Gucy2e(-/-) mice relative to Nrl(-/-) controls. Thus, Nrl(-/-) Gucy2e(-/-) mice possess an expanded dynamic range in ERG response to gene replacement relative to other models. Lastly, we show that a candidate clinical vector, AAV5-GRK1-GUCY2D, when delivered to adult Nrl(-/-) Gucy2e(-/-) mice, restores retinal function that persists for at least 6 months. Our results provide strong support for clinical application of a gene therapy targeted to the cone-rich, central retina of LCA1 patients.
Figures







Similar articles
-
AAV-mediated gene therapy in the guanylate cyclase (RetGC1/RetGC2) double knockout mouse model of Leber congenital amaurosis.Hum Gene Ther. 2013 Feb;24(2):189-202. doi: 10.1089/hum.2012.193. Hum Gene Ther. 2013. PMID: 23210611 Free PMC article.
-
Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency.Hum Gene Ther. 2011 Oct;22(10):1179-90. doi: 10.1089/hum.2011.069. Epub 2011 Aug 10. Hum Gene Ther. 2011. PMID: 21671801 Free PMC article.
-
Functional study of two biochemically unusual mutations in GUCY2D Leber congenital amaurosis expressed via adenoassociated virus vector in mouse retinas.Mol Vis. 2016 Nov 10;22:1342-1351. eCollection 2016. Mol Vis. 2016. PMID: 27881908 Free PMC article.
-
A Mini-review: Animal Models of GUCY2D Leber Congenital Amaurosis (LCA1).Adv Exp Med Biol. 2016;854:253-8. doi: 10.1007/978-3-319-17121-0_34. Adv Exp Med Biol. 2016. PMID: 26427419 Review.
-
Leber congenital amaurosis caused by mutations in GUCY2D.Cold Spring Harb Perspect Med. 2014 Sep 25;5(1):a017350. doi: 10.1101/cshperspect.a017350. Cold Spring Harb Perspect Med. 2014. PMID: 25256176 Free PMC article. Review.
Cited by
-
Animal modelling for inherited central vision loss.J Pathol. 2016 Jan;238(2):300-10. doi: 10.1002/path.4641. Epub 2015 Nov 13. J Pathol. 2016. PMID: 26387748 Free PMC article. Review.
-
Development of an AAV-CRISPR-Cas9-based treatment for dominant cone-rod dystrophy 6.Mol Ther Methods Clin Dev. 2023 Jun 1;30:48-64. doi: 10.1016/j.omtm.2023.05.020. eCollection 2023 Sep 14. Mol Ther Methods Clin Dev. 2023. PMID: 37361352 Free PMC article.
-
Somatic Gene Editing of GUCY2D by AAV-CRISPR/Cas9 Alters Retinal Structure and Function in Mouse and Macaque.Hum Gene Ther. 2019 May;30(5):571-589. doi: 10.1089/hum.2018.193. Epub 2018 Dec 20. Hum Gene Ther. 2019. PMID: 30358434 Free PMC article.
-
Guanylate cyclase-activating protein 2 contributes to phototransduction and light adaptation in mouse cone photoreceptors.J Biol Chem. 2018 May 11;293(19):7457-7465. doi: 10.1074/jbc.RA117.001574. Epub 2018 Mar 16. J Biol Chem. 2018. PMID: 29549122 Free PMC article.
-
Rod Outer Segment Development Influences AAV-Mediated Photoreceptor Transduction After Subretinal Injection.Hum Gene Ther. 2017 Jun;28(6):464-481. doi: 10.1089/hum.2017.020. Hum Gene Ther. 2017. PMID: 28510482 Free PMC article.
References
-
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron 2005;48:387–401 - PubMed
-
- Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci 1996;19:547–554 - PubMed
-
- Shyjan AW, de Sauvage FJ, Gillett NA, et al. . Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron 1992;9:727–737 - PubMed
-
- Margulis A, Goraczniak RM, Duda T, et al. . Structural and biochemical identity of retinal rod outer segment membrane guanylate cyclase. Biochem Biophys Res Commun 1993;194:855–861 - PubMed
-
- Dizhoor AM, Lowe DG, Olshevskaya EV, et al. . The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron 1994;12:1345–1352 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

