Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season
- PMID: 26247435
- PMCID: PMC5779578
- DOI: 10.15585/mmwr.mm6430a3
Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season
Abstract
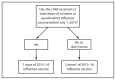
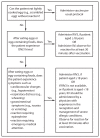
This report updates the 2014 recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding the use of seasonal influenza vaccines. Updated information for the 2015-16 season includes 1) antigenic composition of U.S. seasonal influenza vaccines; 2) information on influenza vaccine products expected to be available for the 2015-16 season; 3) an updated algorithm for determining the appropriate number of doses for children aged 6 months through 8 years; and 4) recommendations for the use of live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) when either is available, including removal of the 2014-15 preferential recommendation for LAIV for healthy children aged 2 through 8 years. Information regarding topics related to influenza vaccination that are not addressed in this report is available in the 2013 ACIP seasonal influenza recommendations.
Figures
References
-
- CDC. Prevention and control of seasonal influenza with vaccines. recommendations of the Advisory Committee on Immunization Practices—United States, 2013–14. MMWR Recomm Rep. 2013;62(RR-7):1–43. - PubMed
-
- Ochiai H, Shibata M, Kamimura K, Niwayama S. Evaluation of the efficacy of split-product trivalent A(H1N1), A(H3N2), and B influenza vaccines: reactogenicity, immunogenicity and persistence of antibodies following two doses of vaccines. Microbiol Immunol. 1986;30:1141–9. - PubMed
-
- Künzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 1996;14:1108–10. - PubMed
-
- Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

