Targeted expression of BikDD combined with metronomic doxorubicin induces synergistic antitumor effect through Bax activation in hepatocellular carcinoma
- PMID: 26247632
- PMCID: PMC4695153
- DOI: 10.18632/oncotarget.4278
Targeted expression of BikDD combined with metronomic doxorubicin induces synergistic antitumor effect through Bax activation in hepatocellular carcinoma
Abstract
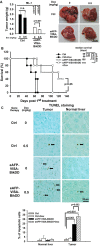
Conventional chemotherapy is commonly used to treat advanced non-resectable hepatocellular carcinoma (HCC) but this treatment modality has not demonstrated convincing survival benefit in HCC patients. Our previous studies indicated that targeted expression of therapeutic BikDD driven by a liver cancer-specific α-fetoprotein promoter/enhancer (eAFP) in the VISA backbone (eAFP-VISA-BikDD) significantly and specifically kills HCC cells in multiple orthotopic animal models. To enhance its therapeutic efficacy, we combined eAFP-VISA-BikDD with chemotherapeutic agents and found that eAFP-VISA-BikDD plus doxorubicin (Dox) or 5-fluorouracil (5-FU) demonstrated synergistic cytotoxicity in HCC cells. Specifically, the combination of eAFP-VISA-BikDD plus Dox markedly induced apoptosis via increased Bax mitochondrial translocation and cytoplasmic cytochrome c release. Compared with either agent alone, a low dose of Dox combined with eAFP-VISA-BikDD induced better antitumor effect and prolonged longer survival of mice in two orthotopic liver cancer xenograft models. Our findings provide strong preclinical support for evaluating the combined therapy of eAFP-VISA-BikDD and Dox in a clinical setting as a treatment option for HCC.
Keywords: combination therapy; hepatocellular carcinoma; metronomic chemotherapy; orthotopic animal model; synergistic antitumor effect.
Conflict of interest statement
Mien-Chie Hung has ownership interest (including patents) for BikDD. All other authors have no conflicts of interest to declare.
Figures





Similar articles
-
Targeted BikDD expression kills androgen-dependent and castration-resistant prostate cancer cells.Mol Cancer Ther. 2014 Jul;13(7):1813-25. doi: 10.1158/1535-7163.MCT-13-1004. Epub 2014 Apr 30. Mol Cancer Ther. 2014. PMID: 24785255 Free PMC article.
-
Targeted expression of BikDD eliminates breast cancer with virtually no toxicity in noninvasive imaging models.Mol Cancer Ther. 2012 Sep;11(9):1915-24. doi: 10.1158/1535-7163.MCT-12-0191. Epub 2012 Jul 2. Mol Cancer Ther. 2012. PMID: 22752427
-
Low-toxicity transferrin-guided polymersomal doxorubicin for potent chemotherapy of orthotopic hepatocellular carcinoma in vivo.Acta Biomater. 2019 Jul 1;92:196-204. doi: 10.1016/j.actbio.2019.05.034. Epub 2019 May 15. Acta Biomater. 2019. PMID: 31102765
-
Novel patient-derived preclinical models of liver cancer.J Hepatol. 2020 Feb;72(2):239-249. doi: 10.1016/j.jhep.2019.09.028. J Hepatol. 2020. PMID: 31954489 Review.
-
Metronomic chemotherapy: possible clinical application in advanced hepatocellular carcinoma.Transl Oncol. 2013 Oct 1;6(5):511-9. doi: 10.1593/tlo.13481. eCollection 2013. Transl Oncol. 2013. PMID: 24151531 Free PMC article. Review.
Cited by
-
Analysis of risk factors of hepatocellular carcinoma and establishment of a clinical prognosis model.Front Oncol. 2023 Mar 22;13:1067353. doi: 10.3389/fonc.2023.1067353. eCollection 2023. Front Oncol. 2023. PMID: 37035138 Free PMC article.
-
Nanoparticle Delivery of miR-34a Eradicates Long-term-cultured Breast Cancer Stem Cells via Targeting C22ORF28 Directly.Theranostics. 2017 Oct 17;7(19):4805-4824. doi: 10.7150/thno.20771. eCollection 2017. Theranostics. 2017. PMID: 29187905 Free PMC article.
-
Combination treatment including targeted therapy for advanced hepatocellular carcinoma.Oncotarget. 2016 Oct 25;7(43):71036-71051. doi: 10.18632/oncotarget.11954. Oncotarget. 2016. PMID: 27626176 Free PMC article. Review.
-
Microvesicles: the functional mediators in sorafenib resistance.Cancer Drug Resist. 2022 Jun 23;5(3):749-761. doi: 10.20517/cdr.2021.137. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36176764 Free PMC article. Review.
References
-
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nature clinical practice Oncology. 2007;4:424–432. - PubMed
-
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. - PubMed
-
- Han KH, Park JY. Chemotherapy for advanced hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2008;23:682–684. - PubMed
-
- Tanikawa K. Chemotherapy for highly advanced hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2001;16:361–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

