Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB
- PMID: 26248044
- PMCID: PMC4527750
- DOI: 10.1371/journal.pone.0134478
Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB
Abstract
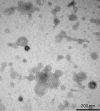
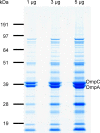
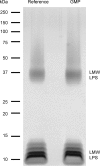
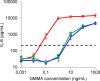
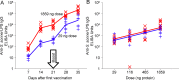
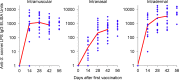
Recently, we developed a high yield production process for outer membrane particles from genetically modified bacteria, called Generalized Modules of Membrane Antigens (GMMA), and the corresponding simple two step filtration purification, enabling economic manufacture of these particles for use as vaccines. Using a Shigella sonnei strain that was genetically modified to produce penta-acylated lipopolysaccharide (LPS) with reduced endotoxicity and to maintain the virulence plasmid encoding for the immunodominant O antigen component of the LPS, scale up of the process to GMP pilot scale was straightforward and gave high yields of GMMA with required purity and consistent results. GMMA were formulated with Alhydrogel and were highly immunogenic in mice and rabbits. In mice, a single immunization containing 29 ng protein and 1.75 ng of O antigen elicited substantial anti-LPS antibody levels. As GMMA contain LPS and lipoproteins, assessing potential reactogenicity was a key aspect of vaccine development. In an in vitro monocyte activation test, GMMA from the production strain showed a 600-fold lower stimulatory activity than GMMA with unmodified LPS. Two in vivo tests confirmed the low potential for reactogenicity. We established a modified rabbit pyrogenicity test based on the European Pharmacopoeia pyrogens method but using intramuscular administration of the full human dose (100 μg of protein). The vaccine elicited an average temperature rise of 0.5°C within four hours after administration, which was considered acceptable and showed that the test is able to detect a pyrogenic response. Furthermore, a repeat dose toxicology study in rabbits using intramuscular (100 μg/dose), intranasal (80 μg/dose), and intradermal (10 μg/dose) administration routes showed good tolerability of the vaccine by all routes and supported its suitability for use in humans. The S. sonnei GMMA vaccine is now in Phase 1 dose-escalation clinical trials.
Conflict of interest statement
Figures



References
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2197–2223. 10.1016/S0140-6736(12)61689-4 - DOI - PubMed
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380: 2095–2128. 10.1016/S0140-6736(12)61728-0 - DOI - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382: 209–222. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

