Lithium Modulates Autophagy in Esophageal and Colorectal Cancer Cells and Enhances the Efficacy of Therapeutic Agents In Vitro and In Vivo
- PMID: 26248051
- PMCID: PMC4527721
- DOI: 10.1371/journal.pone.0134676
Lithium Modulates Autophagy in Esophageal and Colorectal Cancer Cells and Enhances the Efficacy of Therapeutic Agents In Vitro and In Vivo
Abstract
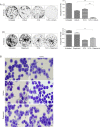
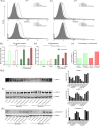
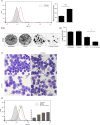
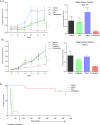
Many epithelial cancers, particularly gastrointestinal tract cancers, remain poor prognosis diseases, due to resistance to cytotoxic therapy and local or metastatic recurrence. We have previously shown that apoptosis incompetent esophageal cancer cells induce autophagy in response to chemotherapeutic agents and this can facilitate their recovery. However, known pharmacological inhibitors of autophagy could not enhance cytotoxicity. In this study, we have examined two well known, clinically approved autophagy inducers, rapamycin and lithium, for their effects on chemosensitivity in apoptosis incompetent cancer cells. Both lithium and rapamycin were shown to induce autophagosomes in esophageal and colorectal cancer cells by western blot analysis of LC3 isoforms, morphology and FACS quantitation of Cyto-ID or mCherry-GFP-LC3. Analysis of autophagic flux indicates inefficient autophagosome processing in lithium treated cells, whereas rapamycin treated cells showed efficient flux. Viability and recovery was assessed by clonogenic assays. When combined with the chemotherapeutic agent 5-fluorouracil, rapamycin was protective. In contrast, lithium showed strong enhancement of non-apoptotic cell death. The combination of lithium with 5-fluorouracil or oxaliplatin was then tested in the syngenic mouse (balb/c) colorectal cancer model--CT26. When either chemotherapeutic agent was combined with lithium a significant reduction in tumor volume was achieved. In addition, survival was dramatically increased in the combination group (p < 0.0001), with > 50% of animals achieving long term cure without re-occurrence (> 1 year tumor free). Thus, combination treatment with lithium can substantially improve the efficacy of chemotherapeutic agents in apoptosis deficient cancer cells. Induction of compromised autophagy may contribute to this cytotoxicity.
Conflict of interest statement
Figures

Similar articles
-
Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death.Cancer Lett. 2020 Jul 1;481:15-23. doi: 10.1016/j.canlet.2020.03.010. Epub 2020 Mar 14. Cancer Lett. 2020. PMID: 32184145
-
Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin.Int J Mol Sci. 2018 Jun 14;19(6):1763. doi: 10.3390/ijms19061763. Int J Mol Sci. 2018. PMID: 29899208 Free PMC article.
-
UBE2L6/UBCH8 and ISG15 attenuate autophagy in esophageal cancer cells.Oncotarget. 2017 Apr 4;8(14):23479-23491. doi: 10.18632/oncotarget.15182. Oncotarget. 2017. PMID: 28186990 Free PMC article.
-
Oxaliplatin: a review of approved uses.Expert Opin Pharmacother. 2012 Jan;13(1):125-37. doi: 10.1517/14656566.2012.643870. Expert Opin Pharmacother. 2012. PMID: 22149372 Review.
-
Pharmacological control of autophagy: therapeutic perspectives in inflammatory bowel disease and colorectal cancer.Curr Pharm Des. 2012;18(26):3853-73. doi: 10.2174/138161212802083653. Curr Pharm Des. 2012. PMID: 22632751 Review.
Cited by
-
Codonopis bulleynana Forest ex Diels inhibits autophagy and induces apoptosis of colon cancer cells by activating the NF-κB signaling pathway.Int J Mol Med. 2018 Mar;41(3):1305-1314. doi: 10.3892/ijmm.2017.3337. Epub 2017 Dec 21. Int J Mol Med. 2018. PMID: 29286074 Free PMC article.
-
Lack of Autophagy Induction by Lithium Decreases Neuroprotective Effects in the Striatum of Aged Rats.Pharmaceutics. 2021 Jan 21;13(2):135. doi: 10.3390/pharmaceutics13020135. Pharmaceutics. 2021. PMID: 33494241 Free PMC article.
-
Lithium in Cancer Therapy: Friend or Foe?Cancers (Basel). 2023 Feb 8;15(4):1095. doi: 10.3390/cancers15041095. Cancers (Basel). 2023. PMID: 36831437 Free PMC article. Review.
-
LiCl Treatment Induces Programmed Cell Death of Schwannoma Cells through AKT- and MTOR-Mediated Necroptosis.Neurochem Res. 2017 Aug;42(8):2363-2371. doi: 10.1007/s11064-017-2256-2. Epub 2017 Apr 10. Neurochem Res. 2017. PMID: 28397069
-
Peiminine inhibits colorectal cancer cell proliferation by inducing apoptosis and autophagy and modulating key metabolic pathways.Oncotarget. 2017 Jul 18;8(29):47619-47631. doi: 10.18632/oncotarget.17411. Oncotarget. 2017. PMID: 28496003 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

