Molecular Cloning and Functional Studies of Two Kazal-Type Serine Protease Inhibitors Specifically Expressed by Nasonia vitripennis Venom Apparatus
- PMID: 26248077
- PMCID: PMC4549731
- DOI: 10.3390/toxins7082888
Molecular Cloning and Functional Studies of Two Kazal-Type Serine Protease Inhibitors Specifically Expressed by Nasonia vitripennis Venom Apparatus
Erratum in
- Toxins (Basel). 2015 Sep;7(9):3636
Abstract
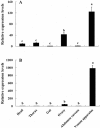
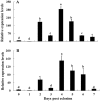
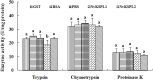
Two cDNA sequences of Kazal-type serine protease inhibitors (KSPIs) in Nasonia vitripennis, NvKSPI-1 and NvKSPI-2, were characterized and their open reading frames (ORFs) were 198 and 264 bp, respectively. Both NvKSPI-1 and NvKSPI-2 contained a typical Kazal-type domain. Real-time quantitative PCR (RT-qPCR) results revealed that NvKSPI-1 and NvKSPI-2 mRNAs were mostly detected specifically in the venom apparatus, while they were expressed at lower levels in the ovary and much lower levels in other tissues tested. In the venom apparatus, both NvKSPI-1 and NvKSPI-2 transcripts were highly expressed on the fourth day post eclosion and then declined gradually. The NvKSPI-1 and NvKSPI-2 genes were recombinantly expressed utilizing a pGEX-4T-2 vector, and the recombinant products fused with glutathione S-transferase were purified. Inhibition of recombinant GST-NvKSPI-1 and GST-NvKSPI-2 to three serine protease inhibitors (trypsin, chymotrypsin, and proteinase K) were tested and results showed that only NvKSPI-1 could inhibit the activity of trypsin. Meanwhile, we evaluated the influence of the recombinant GST-NvKSPI-1 and GST-NvKSPI-2 on the phenoloxidase (PO) activity and prophenoloxidase (PPO) activation of hemolymph from a host pupa, Musca domestica. Results showed PPO activation in host hemolymph was inhibited by both recombinant proteins; however, there was no significant inhibition on the PO activity. Our results suggested that NvKSPI-1 and NvKSPI-2 could inhibit PPO activation in host hemolymph and trypsin activity in vitro.
Keywords: Kazal-type; Nasonia vitripennis; humoral immunity; serine protease inhibitors.
Figures






Similar articles
-
Identification of a small pacifastin protease inhibitor from Nasonia vitripennis venom that inhibits humoral immunity of host (Musca domestica).Toxicon. 2017 Jun 1;131:54-62. doi: 10.1016/j.toxicon.2017.03.005. Epub 2017 Mar 8. Toxicon. 2017. PMID: 28283430
-
Prophenoloxidase from Pieris rapae: gene cloning, activity, and transcription in response to venom/calyx fluid from the endoparasitoid wasp Cotesia glomerata.J Zhejiang Univ Sci B. 2011 Feb;12(2):103-15. doi: 10.1631/jzus.B1000275. J Zhejiang Univ Sci B. 2011. PMID: 21265042 Free PMC article.
-
A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae.Eur J Biochem. 2000 Oct;267(20):6188-96. doi: 10.1046/j.1432-1327.2000.01695.x. Eur J Biochem. 2000. PMID: 11012672
-
Venom proteins of the parasitoid wasp Nasonia vitripennis: recent discovery of an untapped pharmacopee.Toxins (Basel). 2010 Apr;2(4):494-516. doi: 10.3390/toxins2040494. Epub 2010 Mar 30. Toxins (Basel). 2010. PMID: 22069597 Free PMC article. Review.
-
Serine proteinase inhibitors in arthropod immunity.Dev Comp Immunol. 1999 Jun-Jul;23(4-5):291-301. doi: 10.1016/s0145-305x(99)00012-9. Dev Comp Immunol. 1999. PMID: 10426423 Review.
Cited by
-
An Introduction to the Toxins Special Issue on "Bee and Wasp Venoms: Biological Characteristics and Therapeutic Application".Toxins (Basel). 2016 Oct 28;8(11):315. doi: 10.3390/toxins8110315. Toxins (Basel). 2016. PMID: 27801836 Free PMC article.
-
The Pupal Ectoparasitoid Pachycrepoideus vindemmiae Regulates Cellular and Humoral Immunity of Host Drosophila melanogaster.Front Physiol. 2019 Oct 11;10:1282. doi: 10.3389/fphys.2019.01282. eCollection 2019. Front Physiol. 2019. PMID: 31680999 Free PMC article.
-
Host Transcriptome Analysis of Spodoptera frugiperda Larvae Parasitized by Microplitis manilae.Insects. 2023 Jan 17;14(2):100. doi: 10.3390/insects14020100. Insects. 2023. PMID: 36835669 Free PMC article.
-
An integrated transcriptomic and proteomic approach to identify the main Torymus sinensis venom components.Sci Rep. 2021 Mar 3;11(1):5032. doi: 10.1038/s41598-021-84385-5. Sci Rep. 2021. PMID: 33658582 Free PMC article.
-
Innate Immunity in Insects: The Lights and Shadows of Phenoloxidase System Activation.Int J Mol Sci. 2025 Feb 4;26(3):1320. doi: 10.3390/ijms26031320. Int J Mol Sci. 2025. PMID: 39941087 Free PMC article. Review.
References
-
- Friedrich T., Kroger B., Bialojan S., Lemaire H.G., Hoffken H.W., Reuschenbach P., Otte M., Dodt J. A Kazal-type inhibitor with thrombin specificity from Rhodnius prolixus. J. Biol. Chem. 1993;268:16216–16222. - PubMed
-
- Sommerhoff C.P., Sollner C., Mentele R., Piechottka G.P., Auerswald E.A., Fritz H. A Kazal-type inhibitor of human mast cell tryptase: Isolation from the medical leech Hirudo medicinalis, characterization, and sequence analysis. Biol. Chem. Hoppe. Seyler. 1994;375:685–694. doi: 10.1515/bchm3.1994.375.10.685. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

