The central role of muscle stem cells in regenerative failure with aging
- PMID: 26248268
- PMCID: PMC4731230
- DOI: 10.1038/nm.3918
The central role of muscle stem cells in regenerative failure with aging
Abstract
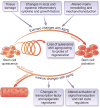
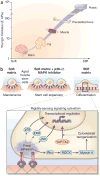
Skeletal muscle mass, function, and repair capacity all progressively decline with aging, restricting mobility, voluntary function, and quality of life. Skeletal muscle repair is facilitated by a population of dedicated muscle stem cells (MuSCs), also known as satellite cells, that reside in anatomically defined niches within muscle tissues. In adult tissues, MuSCs are retained in a quiescent state until they are primed to regenerate damaged muscle through cycles of self-renewal divisions. With aging, muscle tissue homeostasis is progressively disrupted and the ability of MuSCs to repair injured muscle markedly declines. Until recently, this decline has been largely attributed to extrinsic age-related alterations in the microenvironment to which MuSCs are exposed. However, as highlighted in this Perspective, recent reports show that MuSCs also progressively undergo cell-intrinsic alterations that profoundly affect stem cell regenerative function with aging. A more comprehensive understanding of the interplay of stem cell-intrinsic and extrinsic factors will set the stage for improving cell therapies capable of restoring tissue homeostasis and enhancing muscle repair in the aged.
Figures


References
-
- Buchman TG. The community of the self. Nature. 2002;420:246–251. - PubMed
-
- Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–342. - PubMed
-
- Montarras D, L'Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

