Exposure-Response Analyses for Tafenoquine after Administration to Patients with Plasmodium vivax Malaria
- PMID: 26248362
- PMCID: PMC4576039
- DOI: 10.1128/AAC.00718-15
Exposure-Response Analyses for Tafenoquine after Administration to Patients with Plasmodium vivax Malaria
Abstract
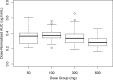
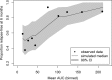
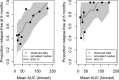
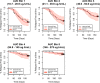
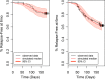
Tafenoquine (TQ), a new 8-aminoquinoline with activity against all stages of the Plasmodium vivax life cycle, is being developed for the radical cure of acute P. vivax malaria in combination with chloroquine. The efficacy and exposure data from a pivotal phase 2b dose-ranging study were used to conduct exposure-response analyses for TQ after administration to subjects with P. vivax malaria. TQ exposure (i.e., area under the concentration-time curve [AUC]) and region (Thailand compared to Peru and Brazil) were found to be statistically significant predictors of clinical response based on multivariate logistic regression analyses. After accounting for region/country, the odds of being relapse free at 6 months increased by approximately 51% (95% confidence intervals [CI], 25%, 82%) for each 25-U increase in AUC above the median value of 54.5 μg · h/ml. TQ exposure was also a significant predictor of the time to relapse of the infection. The final parametric, time-to-event model for the time to relapse, included a Weibull distribution hazard function, AUC, and country as covariates. Based on the model, the risk of relapse decreased by 30% (95% CI, 17% to 42%) for every 25-U increase in AUC. Monte Carlo simulations indicated that the 300-mg dose of TQ would provide an AUC greater than the clinically relevant breakpoint obtained in a classification and regression tree (CART) analysis (56.4 μg · h/ml) in more than 90% of subjects and consequently result in a high probability of being relapse free at 6 months. This model-based approach was critical in selecting an appropriate phase 3 dose. (This study has been registered at ClinicalTrials.gov under registration no. NCT01376167.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- WHO. 2010. Guidelines for the treatment of malaria, 2nd ed WHO, Geneva, Switzerland.
-
- Walsh DS, Looareesuwan S, Wilairatana P, Heppner DG Jr, Tang DB, Brewer TG, Chokejindachai W, Viriyavejakul P, Kyle DE, Milhous WK, Schuster BG, Horton J, Braitman DJ, Brueckner RP. 1999. Randomized dose-ranging study of the safety and efficacy of WR238605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in Thailand. J Infect Dis 180:1282–1287. doi:10.1086/315034. - DOI - PubMed
-
- Walsh DS, Wilairatana P, Tang DB, Heppner DG Jr, Brewer TG, Krudsood S, Silachamroon U, Phumratanaprapin W, Siriyanonda D, Looareesuwan S. 2004. Randomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapse. Clin Infect Dis 39:1095–1103. doi:10.1086/424508. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

