Amikacin Concentrations Predictive of Ototoxicity in Multidrug-Resistant Tuberculosis Patients
- PMID: 26248372
- PMCID: PMC4576092
- DOI: 10.1128/AAC.01050-15
Amikacin Concentrations Predictive of Ototoxicity in Multidrug-Resistant Tuberculosis Patients
Abstract
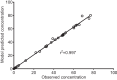
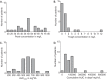
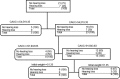
Aminoglycosides, such as amikacin, are used to treat multidrug-resistant tuberculosis. However, ototoxicity is a common problem and is monitored using peak and trough amikacin concentrations based on World Health Organization recommendations. Our objective was to identify clinical factors predictive of ototoxicity using an agnostic machine learning method. We used classification and regression tree (CART) analyses to identify clinical factors, including amikacin concentration thresholds that predicted audiometry-confirmed ototoxicity among 28 multidrug-resistant pulmonary tuberculosis patients in Botswana. Amikacin concentrations were measured for all patients. The quantitative relationship between predictive factors and the probability of ototoxicity were then identified using probit analyses. The primary predictors of ototoxicity on CART analyses were cumulative days of therapy, followed by cumulative area under the concentration-time curve (AUC), which improved on the primary predictor by 87%. The area under the receiver operating curve was 0.97 on the test set. Peak and trough were not predictors in any tree. When algorithms were forced to pick peak and trough as primary predictors, the area under the receiver operating curve fell to 0.46. Probit analysis revealed that the probability of ototoxicity increased sharply starting after 6 months of therapy to near maximum at 9 months. A 10% probability of ototoxicity occurred with a threshold cumulative AUC of 87,232 days · mg · h/liter, while that of 20% occurred at 120,000 days · mg · h/liter. Thus, cumulative amikacin AUC and duration of therapy, and not peak and trough concentrations, should be used as the primary decision-making parameters to minimize the likelihood of ototoxicity in multidrug-resistant tuberculosis.
Copyright © 2015, Modongo et al.
Figures

References
-
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580. doi:10.1016/S0140-6736(06)69573-1. - DOI - PubMed
-
- World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. WHO/HTM/TB/2010.3. 2010. World Health Organization, Geneva, Switzerland.
-
- Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rusch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. 2011. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 38:516–528. doi:10.1183/09031936.00073611. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

