Key Residues of Outer Membrane Protein OprI Involved in Hexamer Formation and Bacterial Susceptibility to Cationic Antimicrobial Peptides
- PMID: 26248382
- PMCID: PMC4576038
- DOI: 10.1128/AAC.01406-15
Key Residues of Outer Membrane Protein OprI Involved in Hexamer Formation and Bacterial Susceptibility to Cationic Antimicrobial Peptides
Abstract
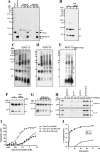
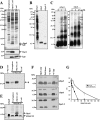
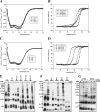
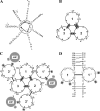
Antimicrobial peptides (AMPs) are important components of the host innate defense mechanism against invading pathogens. Our previous studies have shown that the outer membrane protein, OprI from Pseudomonas aeruginosa or its homologue, plays a vital role in the susceptibility of Gram-negative bacteria to cationic α-helical AMPs (Y. M. Lin, S. J. Wu, T. W. Chang, C. F. Wang, C. S. Suen, M. J. Hwang, M. D. Chang, Y. T. Chen, Y. D. Liao, J Biol Chem 285:8985-8994, 2010, http://dx.doi.org/10.1074/jbc.M109.078725; T. W. Chang, Y. M. Lin, C. F. Wang, Y. D. Liao, J Biol Chem 287:418-428, 2012, http://dx.doi.org/10.1074/jbc.M111.290361). Here, we obtained two forms of recombinant OprI: rOprI-F, a hexamer composed of three disulfide-bridged dimers, was active in AMP binding, while rOprI-R, a trimer, was not. All the subunits predominantly consisted of α-helices and exhibited rigid structures with a melting point centered around 76°C. Interestingly, OprI tagged with Escherichia coli signal peptide was expressed in a hexamer, which was anchored on the surface of E. coli, possibly through lipid acids added at the N terminus of OprI and involved in the binding and susceptibility to AMP as native P. aeruginosa OprI. Deletion and mutation studies showed that Cys1 and Asp27 played a key role in hexamer formation and AMP binding, respectively. The increase of OprI hydrophobicity upon AMP binding revealed that it undergoes conformational changes for membrane fusion. Our results showed that OprI on bacterial surfaces is responsible for the recruitment and susceptibility to amphipathic α-helical AMPs and may be used to screen antimicrobials.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Sarkosyl-Induced Helical Structure of an Antimicrobial Peptide GW-Q6 Plays an Essential Role in the Binding of Surface Receptor OprI in Pseudomonas aeruginosa.PLoS One. 2016 Oct 11;11(10):e0164597. doi: 10.1371/journal.pone.0164597. eCollection 2016. PLoS One. 2016. PMID: 27727309 Free PMC article.
-
Outer membrane protein I of Pseudomonas aeruginosa is a target of cationic antimicrobial peptide/protein.J Biol Chem. 2010 Mar 19;285(12):8985-94. doi: 10.1074/jbc.M109.078725. Epub 2010 Jan 25. J Biol Chem. 2010. PMID: 20100832 Free PMC article.
-
Hydrophobic residues are critical for the helix-forming, hemolytic and bactericidal activities of amphipathic antimicrobial peptide TP4.PLoS One. 2017 Oct 17;12(10):e0186442. doi: 10.1371/journal.pone.0186442. eCollection 2017. PLoS One. 2017. PMID: 29040295 Free PMC article.
-
Anti-microbial peptides: from invertebrates to vertebrates.Immunol Rev. 2004 Apr;198:169-84. doi: 10.1111/j.0105-2896.2004.0124.x. Immunol Rev. 2004. PMID: 15199962 Review.
-
From innate immunity to de-novo designed antimicrobial peptides.Curr Pharm Des. 2002;8(9):715-25. doi: 10.2174/1381612023395367. Curr Pharm Des. 2002. PMID: 11945167 Review.
Cited by
-
Molecular Properties of Virulence and Antibiotic Resistance of Pseudomonas aeruginosa Causing Clinically Critical Infections.Pathogens. 2024 Oct 3;13(10):868. doi: 10.3390/pathogens13100868. Pathogens. 2024. PMID: 39452738 Free PMC article.
-
Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection.Am J Respir Crit Care Med. 2018 Mar 15;197(6):708-727. doi: 10.1164/rccm.201705-1043SO. Am J Respir Crit Care Med. 2018. PMID: 29087211 Free PMC article. Review.
-
Oligomerization and insertion of antimicrobial peptide TP4 on bacterial membrane and membrane-mimicking surfactant sarkosyl.PLoS One. 2019 May 13;14(5):e0216946. doi: 10.1371/journal.pone.0216946. eCollection 2019. PLoS One. 2019. PMID: 31083701 Free PMC article.
-
The FinO/ProQ-like protein PA2582 impacts antimicrobial resistance in Pseudomonas aeruginosa.Front Microbiol. 2024 Jun 26;15:1422742. doi: 10.3389/fmicb.2024.1422742. eCollection 2024. Front Microbiol. 2024. PMID: 39011145 Free PMC article.
-
Sarkosyl-Induced Helical Structure of an Antimicrobial Peptide GW-Q6 Plays an Essential Role in the Binding of Surface Receptor OprI in Pseudomonas aeruginosa.PLoS One. 2016 Oct 11;11(10):e0164597. doi: 10.1371/journal.pone.0164597. eCollection 2016. PLoS One. 2016. PMID: 27727309 Free PMC article.
References
-
- Williams KJ, Bax RP. 2009. Challenges in developing new antibacterial drugs. Curr Opin Investig Drugs 10:157–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

