The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial
- PMID: 26248510
- PMCID: PMC6624136
- DOI: 10.1016/S1473-3099(15)00154-1
The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial
Abstract
Background: Safe and effective vaccines against Ebola could prevent or control outbreaks. The safe use of replication-competent vaccines requires a careful dose-selection process. We report the first safety and immunogenicity results in volunteers receiving 3 × 10(5) plaque-forming units (pfu) of the recombinant vesicular stomatitis virus-based candidate vaccine expressing the Zaire Ebola virus glycoprotein (rVSV-ZEBOV; low-dose vaccinees) compared with 59 volunteers who had received 1 ×10(7) pfu (n=35) or 5 × 10(7) pfu (n=16) of rVSV-ZEBOV (high-dose vaccinees) or placebo (n=8) before a safety-driven study hold.
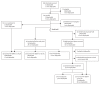
Methods: The Geneva rVSV-ZEBOV study, an investigator-initiated phase 1/2, dose-finding, placebo-controlled, double-blind trial conducted at the University Hospitals of Geneva, Switzerland, enrolled non-pregnant, immunocompetent, and otherwise healthy adults aged 18-65 years. Participants from the low-dose group with no plans to deploy to Ebola-aff5cted regions (non-deployable) were randomised 9:1 in a double-blind fashion using randomly permuted blocks of varying sizes to a single injection of 3 × 10(5) pfu or placebo, whereas deployable participants received single-injection 3 × 10(5) pfu open-label. Primary safety and immunogenicity outcomes were the incidence of adverse events within 14 days of vaccination and day-28 antibody titres, respectively, analysed by intention to treat. After viral oligoarthritis was observed in 11 of the first 51 vaccinees (22%) receiving 10(7) or 5 × 10(7) pfu, 56 participants were given a lower dose (3 × 10(5) pfu, n=51) or placebo (n=5) to assess the effect of dose reduction on safety and immunogenicity. This trial is ongoing with a follow-up period of 12 months; all reported results are from interim databases. This study is registered with ClinicalTrials.gov, number NCT02287480.
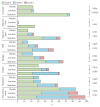
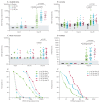
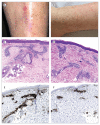
Findings: Between Jan 5 and Jan 26, 2015, 43 non-deployable participants received low-dose rVSV-ZEBOV (3 × 10(5) pfu) or placebo in a double-blind fashion, whereas 13 deployable participants received 3 × 10(5) pfu open-label. Altogether, in the low-dose group, 51 participants received rVSV-ZEBOV and five received placebo. No serious adverse events occurred. At 3 × 10(5) pfu, early-onset reactogenicity remained frequent (45 [88%] of 51 compared with 50 [98%] of 51 high dose and two [15%] of 13 placebo recipients), but mild. Objective fever was present in one (2%) of 51 low-dose versus 13 (25%) of 51 high-dose vaccinees receiving at least 1 ×10(7) pfu (p<0·0001). Subjective fever (p<0·0001), myalgia (p=0·036), and chills (p=0·026) were significantly reduced and their time of onset delayed, reflecting significantly lower viraemia (p<0·0001) and blood monocyte-activation patterns (p=0·0233). Although seropositivity rates remained similarly high (48 [94%] of 51), day-28 EBOV-glycoprotein-binding and neutralising antibody titres were lower in low-dose versus high-dose vaccinees (geometric mean titres 344·5 [95% CI 229·7-516·4] vs 1064·2 [757·6-1495·1]; p<0·0001; and 35·1 [24·7-50·7] vs 127·0 [86·0-187·6]; p<0·0001, respectively). Furthermore, oligoarthritis again occurred on day 10 (median; IQR 9-14) in 13 (25%) of 51 low-dose vaccinees, with maculopapular, vesicular dermatitis, or both in seven (54%) of 13; arthritis was associated with increasing age in low-dose but not high-dose vaccinees. Two vaccinees presented with purpura of the lower legs; histological findings indicated cutaneous vasculitis. The presence of rVSV in synovial fluid and skin lesions confirmed causality.
Interpretation: Reducing the dose of rVSV-ZEBOV improved its early tolerability but lowered antibody responses and did not prevent vaccine-induced arthritis, dermatitis, or vasculitis. Like its efficacy, the safety of rVSV-ZEBOV requires further definition in the target populations of Africa.
Funding: Wellcome Trust through WHO.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests.
Figures
Comment in
-
Use of low dose rVSV-ZEBOV: safety issues in a Swiss cohort.Lancet Infect Dis. 2015 Oct;15(10):1117-1119. doi: 10.1016/S1473-3099(15)00222-4. Epub 2015 Aug 4. Lancet Infect Dis. 2015. PMID: 26248511 No abstract available.
References
-
- WHO. WHO Situation Report. [accessed June 22, 2015]; http://apps.who.int/ebola/currentsituation/ebola-situation-report-17-jun....
-
- Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

