Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1
- PMID: 26248546
- PMCID: PMC4688136
- DOI: 10.1002/hep.28111
Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1
Abstract
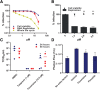
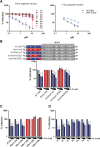
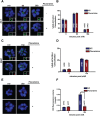
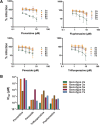
To explore mechanisms of hepatitis C viral (HCV) replication we screened a compound library including licensed drugs. Flunarizine, a diphenylmethylpiperazine used to treat migraine, inhibited HCV cell entry in vitro and in vivo in a genotype-dependent fashion. Analysis of mosaic viruses between susceptible and resistant strains revealed that E1 and E2 glycoproteins confer susceptibility to flunarizine. Time of addition experiments and single particle tracking of HCV demonstrated that flunarizine specifically prevents membrane fusion. Related phenothiazines and pimozide also inhibited HCV infection and preferentially targeted HCV genotype 2 viruses. However, phenothiazines and pimozide exhibited improved genotype coverage including the difficult to treat genotype 3. Flunarizine-resistant HCV carried mutations within the alleged fusion peptide and displayed cross-resistance to these compounds, indicating that these drugs have a common mode of action.
Conclusion: These observations reveal novel details about HCV membrane fusion; moreover, flunarizine and related compounds represent first-in-class HCV fusion inhibitors that merit consideration for repurposing as a cost-effective component of HCV combination therapies.
© 2015 by the American Association for the Study of Liver Diseases.
Figures



Comment in
-
Flunarizine arrests hepatitis C virus membrane fusion.Hepatology. 2016 Jan;63(1):14-6. doi: 10.1002/hep.28224. Epub 2015 Oct 30. Hepatology. 2016. PMID: 26389725 Free PMC article. No abstract available.
References
-
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41‐52. - PubMed
-
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014;146:1176‐1192. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology 2013;57:1333‐1342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

