The new biology of diabetes
- PMID: 26248647
- PMCID: PMC4591190
- DOI: 10.1007/s00125-015-3722-5
The new biology of diabetes
Erratum in
-
Erratum to: The new biology of diabetes.Diabetologia. 2015 Nov;58(11):2683. doi: 10.1007/s00125-015-3758-6. Diabetologia. 2015. PMID: 26376798 No abstract available.
Abstract
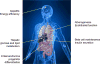
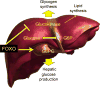
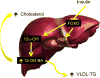
Until recently, type 2 diabetes was seen as a disease caused by an impaired ability of insulin to promote the uptake and utilisation of glucose. Work on forkhead box protein O (FOXO) transcription factors revealed new aspects of insulin action that have led us to articulate a liver- and beta cell-centric narrative of diabetes pathophysiology and treatment. FOXO integrate a surprisingly diverse subset of biological functions to promote metabolic flexibility. In the liver, they controls the glucokinase/glucose-6-phosphatase switch and bile acid pool composition, directing carbons to glucose or lipid utilisation, thus providing a unifying mechanism for the two abnormalities of the diabetic liver: excessive glucose production and increased lipid synthesis and secretion. Moreover, FOXO are necessary to maintain beta cell differentiation, and diabetes development is associated with a gradual loss of FOXO function that brings about beta cell dedifferentiation. We proposed that dedifferentiation is the main cause of beta cell failure and conversion into non-beta endocrine cells, and that treatment should restore beta cell differentiation. Our studies investigating these proposals have revealed new dimensions to the pathophysiology of diabetes that can be leveraged to design new therapies.
Keywords: Beta cell failure; Bile acid; Cholesterol; Dedifferentiation; Diabetes outcome; FOXO; Heart disease; Hepatic glucose production; Lipoprotein; Notch 1; Review.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures




References
-
- Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes. 2004;53:1633–1642. - PubMed
-
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. - PubMed
-
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. - PubMed
-
- Chen KK, Anderson RC. The toxicity and general pharmacology of N1-p-chlorophenyl-N5-isopropyl biguanide. The Journal of pharmacology and experimental therapeutics. 1947;91:157–160. - PubMed
-
- Baggio LL, Drucker DJ. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med. 2006;57:265–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

