Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity
- PMID: 26248660
- PMCID: PMC4722243
- DOI: 10.1038/ijo.2015.145
Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity
Abstract
Background/objectives: Although obesity is associated with low-grade inflammation and metabolic disorders, clinical studies suggested some obese people were metabolically healthy with smaller adipocyte size compared with metabolically abnormal obese (MAO). This indicated adipocyte size may be an important predictor underlay the distinction between MAO and metabolically healthy obese. As recent study has shown that adipocytes expressed class II major histocompatibility complex (MHCII), which functioned as APCs during obesity. However, the relationship between adipocyte hypertrophy and MHCII expression was not involved. Here we hypothesize that hypertrophic adipocytes could be associated with upregulating MHCII to influence adipose tissue metabolism.
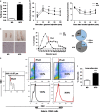
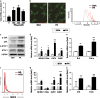
Methods: Adipocytes were sorted by fluorescence-activated cell sorting (FACS) according to the cell size from MAO mice. The activation of MHCII, T cells and related signaling molecules were examined by FACS, ELISA and western blotting. 3T3-L1 cell line and primary adipocytes were used to examine the effect of free fatty acids (FFA) on adipocytes enlargement and MHCII expression.
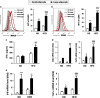
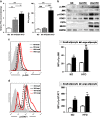
Results: MAO mice had a significant increase in adipocytes size and FFA concentration. The large adipocytes from both obese and non-obese mice expressed higher levels of MHCII than small adipocytes. Importantly, large adipocytes from obese mice stimulated CD4(+) T cells to secrete more interferon (IFN)-γ. Furthermore, the activation of the JNK-STAT1 pathway was involved in upregulation of MHCII in large adipocytes. In vitro FFA treatment promoted adipocyte hypertrophy and expression of MHCII-associated genes.
Conclusions: This study demonstrates that large adipocytes highly express MHCII and function as APC to stimulate IFN-γ-expressing CD4(+) T cells, in which FFA may have important roles before IFN-γ elevated. These findings suggest that adipocyte hypertrophy, rather than overall obesity, is the major contributor to adipose tissue inflammation and insulin resistance.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

