Xanthomonas campestris cell-cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan
- PMID: 26248667
- PMCID: PMC4623683
- DOI: 10.1093/jxb/erv377
Xanthomonas campestris cell-cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan
Abstract
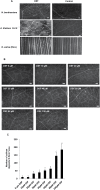
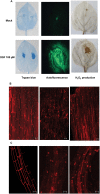
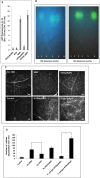
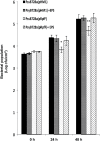
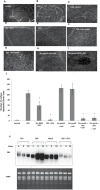
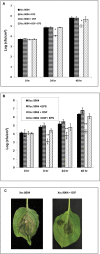
Several secreted and surface-associated conserved microbial molecules are recognized by the host to mount the defence response. One such evolutionarily well-conserved bacterial process is the production of cell-cell signalling molecules which regulate production of multiple virulence functions by a process known as quorum sensing. Here it is shown that a bacterial fatty acid cell-cell signalling molecule, DSF (diffusible signal factor), elicits innate immunity in plants. The DSF family of signalling molecules are highly conserved among many phytopathogenic bacteria belonging to the genus Xanthomonas as well as in opportunistic animal pathogens. Using Arabidopsis, Nicotiana benthamiana, and rice as model systems, it is shown that DSF induces a hypersensitivity reaction (HR)-like response, programmed cell death, the accumulation of autofluorescent compounds, hydrogen peroxide production, and the expression of the PATHOGENESIS-RELATED1 (PR-1) gene. Furthermore, production of the DSF signalling molecule in Pseudomonas syringae, a non-DSF-producing plant pathogen, induces the innate immune response in the N. benthamiana host plant and also affects pathogen growth. By pre- and co-inoculation of DSF, it was demonstrated that the DSF-induced plant defence reduces disease severity and pathogen growth in the host plant. In this study, it was further demonstrated that wild-type Xanthomonas campestris suppresses the DSF-induced innate immunity by secreting xanthan, the main component of extracellular polysaccharide. The results indicate that plants have evolved to recognize a widely conserved bacterial communication system and may have played a role in the co-evolution of host recognition of the pathogen and the communication machinery.
Keywords: Defence suppressor; Nicotiana benthamiana; Xanthomonas campestris pv. campestris; Xanthomonas oryzae pv. oryzae.; elicitor; extracellular polysaccharide; innate immunity; quorum sensing; rice; virulence.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
-
- Adam L, Somerville SC. 1996. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana . The Plant Journal 9, 341–356. - PubMed
-
- Aslam SN, Newman MA, Erbs G, et al. 2008. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Current Biology 18, 1078–1083. - PubMed
-
- Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Dow JM, Williams P, Daniels MJ. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Molecular Microbiology 24, 555–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

