Nanoparticles and clinically applicable cell tracking
- PMID: 26248872
- PMCID: PMC4730978
- DOI: 10.1259/bjr.20150375
Nanoparticles and clinically applicable cell tracking
Abstract
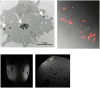
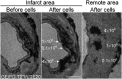
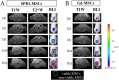
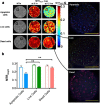
In vivo cell tracking has emerged as a much sought after tool for design and monitoring of cell-based treatment strategies. Various techniques are available for pre-clinical animal studies, from which much has been learned and still can be learned. However, there is also a need for clinically translatable techniques. Central to in vivo cell imaging is labelling of cells with agents that can give rise to signals in vivo, that can be detected and measured non-invasively. The current imaging technology of choice for clinical translation is MRI in combination with labelling of cells with magnetic agents. The main challenge encountered during the cell labelling procedure is to efficiently incorporate the label into the cell, such that the labelled cells can be imaged at high sensitivity for prolonged periods of time, without the labelling process affecting the functionality of the cells. In this respect, nanoparticles offer attractive features since their structure and chemical properties can be modified to facilitate cellular incorporation and because they can carry a high payload of the relevant label into cells. While these technologies have already been applied in clinical trials and have increased the understanding of cell-based therapy mechanism, many challenges are still faced.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

