Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile
- PMID: 26249148
- PMCID: PMC4833190
- DOI: 10.1002/eji.201445404
Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile
Abstract
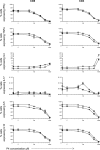
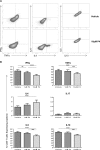
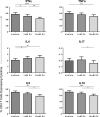
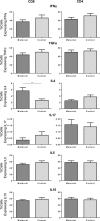
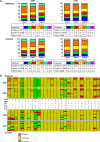
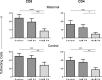
Progesterone is a steroid hormone essential for the maintenance of human pregnancy, and its actions are thought to include promoting maternal immune tolerance of the semiallogenic fetus. We report that exposure of maternal T cells to progesterone at physiological doses induced a unique skewing of the cytokine production profile of CD4(+) and CD8(+) T cells, with reductions not only in potentially deleterious IFN-γ and TNF-α production but also in IL-10 and IL-5. Conversely, production of IL-4 was increased. Maternal T cells also became less polyfunctional, focussing cytokine production toward profiles including IL-4. This was accompanied by reduced T-cell proliferation. Using fetal and viral antigen-specific CD8(+) T-cell clones, we confirmed that this as a direct, nonantigen-specific effect. Yet human T cells lacked conventional nuclear progesterone receptors, implicating a membrane progesterone receptor. CD4(+) and CD8(+) T cells responded to progesterone in a dose-dependent manner, with subtle effects at concentrations comparable to those in maternal blood, but profound effects at concentrations similar to those at the maternal-fetal interface. This characterization of how progesterone modulates T-cell function is important in understanding the normal biology of pregnancy and informing the rational use of progesterone therapy in pregnancies at risk of fetal loss.
Keywords: Human; IL-4; Maternal-fetal tolerance; Progesterone; T cell.
© 2015 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Lissauer, D. M. , Piper, K. P. , Moss, P. A. and Kilby, M. D. , Fetal microchimerism: the cellular and immunological legacy of pregnancy. Expert Rev. Mol. Med. 2009. 11: e33. - PubMed
-
- Tafuri, A. , Alferink, J. , Moller, P. , Hammerling, G. J. and Arnold, B. , T cell awareness of paternal alloantigens during pregnancy. Science 1995. 270: 630–633. - PubMed
-
- Lissauer, D. , Piper, K. , Goodyear, O. , Kilby, M. D. and Moss, P. A. , Fetal‐specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 2012. 189: 1072–1080. - PubMed
-
- Piper, K. P. , McLarnon, A. , Arrazi, J. , Horlock, C. , Ainsworth, J. , Kilby, M. D. , Martin, W. L. et al., Functional HY‐specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol. Reprod. 2007. 76: 96–101. - PubMed
-
- Lissauer, D. , Choudhary, M. , Pachnio, A. , Goodyear, O. , Moss, P. A. and Kilby, M. D. , Cytomegalovirus sero positivity dramatically alters the maternal CD8+ T cell repertoire and leads to the accumulation of highly differentiated memory cells during human pregnancy. Hum. Reprod. 2011. 26: 3355–3365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

