Region-specific disturbed iron distribution in early idiopathic Parkinson's disease measured by quantitative susceptibility mapping
- PMID: 26249218
- PMCID: PMC6869507
- DOI: 10.1002/hbm.22928
Region-specific disturbed iron distribution in early idiopathic Parkinson's disease measured by quantitative susceptibility mapping
Abstract
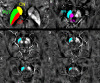
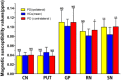
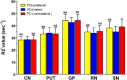
In Parkinson's disease (PD), iron elevation in specific brain regions as well as selective loss of dopaminergic neurons is a major pathologic feature. A reliable quantitative measure of iron deposition is a potential biomarker for PD and may contribute to the investigation of iron-mediated PD. The primary purpose of this study is to assess iron variations in multiple deep grey matter nuclei in early PD with a novel MRI technique, quantitative susceptibility mapping (QSM). The inter-group differences of susceptibility and R2* value in deep grey matter nuclei, namely head of caudate nucleus (CN), putamen (PUT), global pallidus (GP), substantia nigra (SN), and red nucleus (RN), and the correlations between regional iron deposition and the clinical features were explored in forty-four early PD patients and 35 gender and age-matched healthy controls. Susceptibility values were found to be elevated within bilateral SN and RN contralateral to the most affected limb in early PD compared with healthy controls (HCs). The finding of increased susceptibility in bilateral SN is consistent with work on a subgroup of patients at the earliest clinical detectable state (Hoehn and Yahr [1967]: Neurology 17:427-442; Stage I). However, increased R2* values were only seen within SN contralateral to the most affected limb in the PD group when compared with controls. Furthermore, bilateral SN magnetic susceptibility positively correlated with disease duration and UPDRS-III scores in early PD. This finding supports the potential value of QSM as a non-invasive quantitative biomarker of early PD.
Keywords: deep grey matter nuclei; early Parkinson's disease; iron deposition; magnetic resonance imaging; quantitative susceptibility mapping.
© 2015 Wiley Periodicals, Inc.
Figures



Similar articles
-
Iron deposition in Parkinson's disease by quantitative susceptibility mapping.BMC Neurosci. 2019 May 22;20(1):23. doi: 10.1186/s12868-019-0505-9. BMC Neurosci. 2019. PMID: 31117957 Free PMC article.
-
Quantitative Susceptibility Mapping in Parkinson's Disease.PLoS One. 2016 Sep 6;11(9):e0162460. doi: 10.1371/journal.pone.0162460. eCollection 2016. PLoS One. 2016. PMID: 27598250 Free PMC article.
-
Quantifying iron deposition within the substantia nigra of Parkinson's disease by quantitative susceptibility mapping.J Neurol Sci. 2018 Mar 15;386:46-52. doi: 10.1016/j.jns.2018.01.008. Epub 2018 Jan 12. J Neurol Sci. 2018. PMID: 29406966
-
Effectiveness of QSM over R2* in assessment of parkinson's disease - A systematic review.Neurol India. 2020 Mar-Apr;68(2):278-281. doi: 10.4103/0028-3886.284377. Neurol India. 2020. PMID: 32415005
-
Simultaneous Increase of Mean Susceptibility and Mean Kurtosis in the Substantia Nigra as an MRI Neuroimaging Biomarker for Early-Stage Parkinson's Disease: A Systematic Review and Meta-Analysis.J Magn Reson Imaging. 2025 Apr;61(4):1797-1809. doi: 10.1002/jmri.29569. Epub 2024 Aug 29. J Magn Reson Imaging. 2025. PMID: 39210501
Cited by
-
Non-Motor Symptom Burdens Are Not Associated with Iron Accumulation in Early Parkinson's Disease: a Quantitative Susceptibility Mapping Study.J Korean Med Sci. 2018 Mar 26;33(13):e96. doi: 10.3346/jkms.2018.33.e96. J Korean Med Sci. 2018. PMID: 29573246 Free PMC article.
-
Diffusion tensor image analysis along the perivascular space and quantitative susceptibility mapping in the diagnosis and severity assessment of Parkinson's disease.Quant Imaging Med Surg. 2025 Feb 1;15(2):1411-1424. doi: 10.21037/qims-24-1605. Epub 2025 Jan 22. Quant Imaging Med Surg. 2025. PMID: 39995711 Free PMC article.
-
High-resolution mapping of substantia nigra in Parkinson's disease using 7 tesla magnetic resonance imaging.NPJ Parkinsons Dis. 2025 May 6;11(1):113. doi: 10.1038/s41531-025-00972-7. NPJ Parkinsons Dis. 2025. PMID: 40328786 Free PMC article.
-
Impact of high iron intake on cognition and neurodegeneration in humans and in animal models: a systematic review.Nutr Rev. 2017 Jun 1;75(6):456-470. doi: 10.1093/nutrit/nux015. Nutr Rev. 2017. PMID: 28505363 Free PMC article.
-
Combined MR Volumetry and T2* Relaxometry Reveals the Olfactory System as an Iron-Dependent Structure Affected by Radiation.Neurol Int. 2025 Apr 8;17(4):53. doi: 10.3390/neurolint17040053. Neurol Int. 2025. PMID: 40278424 Free PMC article.
References
-
- Andersen JK (2004): Oxidative stress in neurodegeneration: Cause or consequence? Nat Med 10(Suppl):S18–S25. - PubMed
-
- Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI (2013): Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol 73:554–559. - PubMed
-
- Barnham KJ, Masters CL, Bush AI (2004): Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discover 3:205–214. - PubMed
-
- Beck AT, Steer R, Brown G, Manual for the BDI-II (1996): San Antonio, TX: The Psychological Corporation. pp 1–38.
-
- Ben‐Shachar D, Riederer P, Youdim MB (1991): Iron‐melanin interaction and lipid peroxidation: implications for Parkinson's disease. J Neurochem 57:1609–1614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

