Peripheral blood derived mononuclear cells enhance osteoarthritic human chondrocyte migration
- PMID: 26249339
- PMCID: PMC4528856
- DOI: 10.1186/s13075-015-0709-z
Peripheral blood derived mononuclear cells enhance osteoarthritic human chondrocyte migration
Abstract
Introduction: A major problem in cartilage repair is the lack of chondrogenic cells migrating from healthy tissue into defects. Cartilage is essentially avascular and therefore its healing is not considered to involve mononuclear cells. Peripheral blood derived mononuclear cells (PBMC) offer a readily available autologous cell source for clinical use and therefore this study was designed to evaluate the effects of PBMCs on chondrocytes and cartilage.
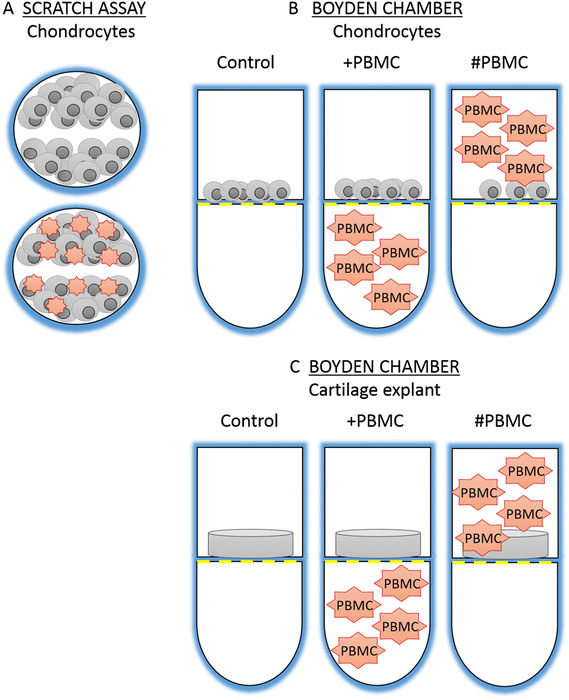
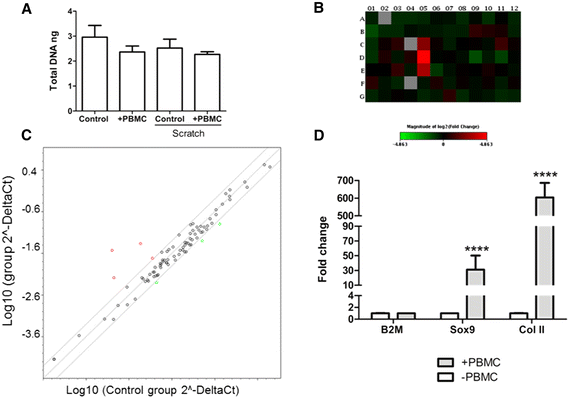
Methods: Human primary chondrocytes and cartilage tissue explants were taken from patients undergoing total knee replacement (n = 17). Peripheral blood samples were obtained from healthy volunteers (n = 12) and mononuclear cells were isolated by density-gradient centrifugation. Cell migration and chemokinetic potential were measured using a scratch assay, xCELLigence and CyQuant assay. PCR array and quantitative PCR was used to evaluate mRNA expression of 87 cell motility and/or chondrogenic genes.
Results: The chondrocyte migration rate was 2.6 times higher at 3 hour time point (p < 0.0001) and total number of migrating chondrocytes was 9.7 times higher (p < 0.0001) after three day indirect PBMC stimulus and 8.2 times higher (p < 0.0001) after three day direct co-culture with PBMCs. A cartilage explant model confirmed that PBMCs also exert a chemokinetic role on ex vivo tissue. PBMC stimulation was found to significantly upregulate the mRNA levels of 2 chondrogenic genes; collagen type II (COL2A1 600-fold, p < 0.0001) and SRY box 9 (SOX9 30-fold, p < 0.0001) and the mRNA levels of 7 genes central in cell motility and migration were differentially regulated by 24h PBMC stimulation.
Conclusion: The results support the concept that PBMC treatment enhances chondrocyte migration without suppressing the chondrogenic phenotype possibly via mechanistic pathways involving MMP9 and IGF1. In the future, peripheral blood mononuclear cells could be used as an autologous point-ofcare treatment to attract native chondrocytes from the diseased tissue to aid in cartilage repair.
Figures

Comment in
-
Regenerative medicine. PBMCs stimulate chondrocyte migration and cartilage repair.Nat Rev Rheumatol. 2015 Oct;11(10):563. doi: 10.1038/nrrheum.2015.118. Epub 2015 Aug 25. Nat Rev Rheumatol. 2015. PMID: 26303955 No abstract available.
References
-
- Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

