MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis
- PMID: 26249667
- PMCID: PMC4528380
- DOI: 10.1186/s13075-015-0723-1
MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis
Abstract
Introduction: Approximately 30% of juvenile idiopathic arthritis (JIA) patients fail to respond to anti-TNF treatment. When clinical remission is induced, some patients relapse after treatment has been stopped. We tested the predictive value of MRP8/14 serum levels to identify responders to treatment and relapse after discontinuation of therapy.
Methods: Samples from 88 non-systemic JIA patients who started and 26 patients who discontinued TNF-blockers were analyzed. MRP8/14 serum levels were measured by in-house MRP8/14 ELISA and by Bühlmann Calprotectin ELISA at start of anti-TNF treatment, within 6 months after start and at discontinuation of etanercept in clinical remission. Patients were categorized into responders (ACRpedi ≥ 50 and/or inactive disease) and non-responders (ACRpedi < 50) within six months after start, response was evaluated by change in JADAS-10. Disease activity was assessed within six months after discontinuation.
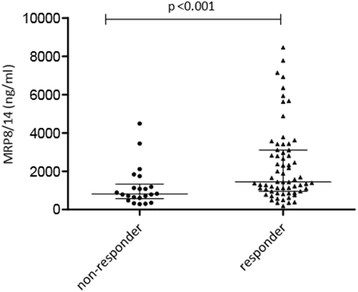
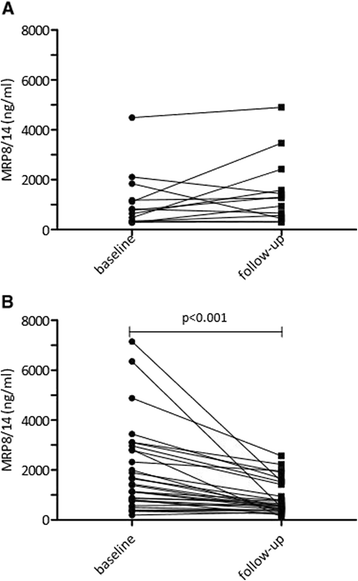
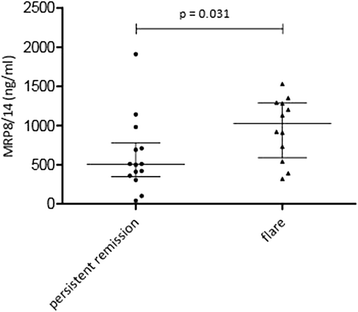
Results: Baseline MRP8/14 levels were higher in responders (median MRP8/14 of 1466 ng/ml (IQR 1045-3170)) compared to non-responders (median MRP8/14 of 812 (IQR 570-1178), p < 0.001). Levels decreased after start of treatment only in responders (p < 0.001). Change in JADAS-10 was correlated with baseline MRP8/14 levels (Spearman's rho 0.361, p = 0.001). Patients who flared within 6 months after treatment discontinuation had higher MRP8/14 levels (p = 0.031, median 1025 ng/ml (IQR 588-1288)) compared to patients with stable remission (505 ng/ml (IQR 346-778)). Results were confirmed by Bühlmann ELISA with high reproducibility but different overall levels.
Conclusion: High levels of baseline MRP8/14 are associated with good response to anti-TNF treatment, whereas elevated MRP8/14 levels at discontinuation of etanercept are associated with higher chance to flare.
Figures
References
-
- Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. Pediatric Rheumatology Collaborative Study G: Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–504. doi: 10.1002/art.23427. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

