How the embryo makes a limb: determination, polarity and identity
- PMID: 26249743
- PMCID: PMC4580101
- DOI: 10.1111/joa.12361
How the embryo makes a limb: determination, polarity and identity
Abstract
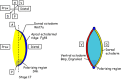
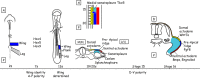
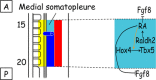
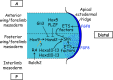
The vertebrate limb with its complex anatomy develops from a small bud of undifferentiated mesoderm cells encased in ectoderm. The bud has its own intrinsic polarity and can develop autonomously into a limb without reference to the rest of the embryo. In this review, recent advances are integrated with classical embryology, carried out mainly in chick embryos, to present an overview of how the embryo makes a limb bud. We will focus on how mesoderm cells in precise locations in the embryo become determined to form a limb and express the key transcription factors Tbx4 (leg/hindlimb) or Tbx5 (wing/forelimb). These Tbx transcription factors have equivalent functions in the control of bud formation by initiating a signalling cascade involving Wnts and fibroblast growth factors (FGFs) and by regulating recruitment of mesenchymal cells from the coelomic epithelium into the bud. The mesoderm that will form limb buds and the polarity of the buds is determined with respect to both antero-posterior and dorso-ventral axes of the body. The position in which a bud develops along the antero-posterior axis of the body will also determine its identity - wing/forelimb or leg/hindlimb. Hox gene activity, under the influence of retinoic acid signalling, is directly linked with the initiation of Tbx5 gene expression in the region along the antero-posterior axis of the body that will form wings/forelimbs and determines antero-posterior polarity of the buds. In contrast, Tbx4 expression in the regions that will form legs/hindlimbs is regulated by the homeoprotein Pitx1 and there is no evidence that Hox genes determine antero-posterior polarity of the buds. Bone morphogenetic protein (BMP) signalling determines the region along the dorso-ventral axis of the body in which both wings/forelimbs and legs/hindlimbs develop and dorso-ventral polarity of the buds. The polarity of the buds leads to the establishment of signalling regions - the dorsal and ventral ectoderm, producing Wnts and BMPs, respectively, the apical ectodermal ridge producing fibroblast growth factors and the polarizing region, Sonic hedgehog (Shh). These signals are the same in both wings/forelimbs and legs/hindlimbs and control growth and pattern formation by providing the mesoderm cells of the limb bud as it develops with positional information. The precise anatomy of the limb depends on the mesoderm cells in the developing bud interpreting positional information according to their identity - determined by Pitx1 in hindlimbs - and genotype. The competence to form a limb extends along the entire antero-posterior axis of the trunk - with Hox gene activity inhibiting the formation of forelimbs in the interlimb region - and also along the dorso-ventral axis.
Keywords: Hox genes; Pitx1; Sonic hedgehog; Tbx4/5; Wnts; antero-posterior polarity; apical ectodermal ridge; bone morphogenetic proteins; dorso-ventral polarity; embryo; fibroblast growth factors; lateral plate mesoderm; limb; limb bud; polarizing region; retinoic acid.
© 2015 Anatomical Society.
Figures


Similar articles
-
Expression of genes encoding bone morphogenetic proteins and sonic hedgehog in talpid (ta3) limb buds: their relationships in the signalling cascade involved in limb patterning.Dev Dyn. 1995 Jun;203(2):187-97. doi: 10.1002/aja.1002030207. Dev Dyn. 1995. PMID: 7655081
-
Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function.Dev Biol. 2001 Aug 15;236(2):421-35. doi: 10.1006/dbio.2001.0346. Dev Biol. 2001. PMID: 11476582
-
The limb field mesoderm determines initial limb bud anteroposterior asymmetry and budding independent of sonic hedgehog or apical ectodermal gene expressions.Development. 1996 Aug;122(8):2319-30. doi: 10.1242/dev.122.8.2319. Development. 1996. PMID: 8756277
-
[Control of the positioning of the vertebrate limb axes during development].Morphologie. 2000 Jun;84(265):17-23. Morphologie. 2000. PMID: 11048294 Review. French.
-
Vertebrate limb development and malformations.Pediatr Res. 1999 Sep;46(3):247-54. doi: 10.1203/00006450-199909000-00001. Pediatr Res. 1999. PMID: 10473037 Review.
Cited by
-
Limb patterning genes and heterochronic development of the emu wing bud.Evodevo. 2016 Dec 20;7:26. doi: 10.1186/s13227-016-0063-5. eCollection 2016. Evodevo. 2016. PMID: 28031782 Free PMC article.
-
Fetal Thigh Circumference Nomograms Across Gestational Ages: A Retrospective Study.J Pers Med. 2025 Jun 22;15(7):265. doi: 10.3390/jpm15070265. J Pers Med. 2025. PMID: 40710382 Free PMC article.
-
Contribution of neural crest-derived stem cells and nasal chondrocytes to articular cartilage regeneration.Cell Mol Life Sci. 2020 Dec;77(23):4847-4859. doi: 10.1007/s00018-020-03567-y. Epub 2020 Jun 5. Cell Mol Life Sci. 2020. PMID: 32504256 Free PMC article. Review.
-
Distal Limb Patterning Requires Modulation of cis-Regulatory Activities by HOX13.Cell Rep. 2016 Dec 13;17(11):2913-2926. doi: 10.1016/j.celrep.2016.11.039. Cell Rep. 2016. PMID: 27974206 Free PMC article.
-
Congenital dyserythropoietic anemia type I: First report from the Congenital Dyserythropoietic Anemia Registry of North America (CDAR).Blood Cells Mol Dis. 2021 Mar;87:102534. doi: 10.1016/j.bcmd.2020.102534. Epub 2020 Dec 24. Blood Cells Mol Dis. 2021. PMID: 33401150 Free PMC article. Clinical Trial.
References
-
- Agarwal P, Wylie JN, Galceran J, et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. - PubMed
-
- Ahn K, Mishina Y, Hanks MC, et al. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. - PubMed
-
- Alam MR, Lee JI, Lee HB, et al. Supernumerary ectopic limbs in Korean indigenous cattle: four case reports. Vet Med. 2007;52:202–206.
-
- Altabef M, Clarke JDW, Tickle C. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development. 1997;124:4547–4556. - PubMed
-
- Altabef M, Logan C, Tickle C, et al. Engrailed-1 misexpression in chick embryos prevents apical ridge formation but preserves segregation of dorsal and ventral ectodermal compartments. Dev Biol. 2000;222:307–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

