Heparan sulfation is essential for the prevention of cellular senescence
- PMID: 26250908
- PMCID: PMC5072436
- DOI: 10.1038/cdd.2015.107
Heparan sulfation is essential for the prevention of cellular senescence
Abstract
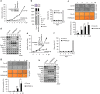
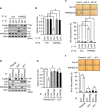
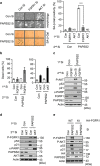
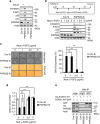
Cellular senescence is considered as an important tumor-suppressive mechanism. Here, we demonstrated that heparan sulfate (HS) prevents cellular senescence by fine-tuning of the fibroblast growth factor receptor (FGFR) signaling pathway. We found that depletion of 3'-phosphoadenosine 5'-phosphosulfate synthetase 2 (PAPSS2), a synthetic enzyme of the sulfur donor PAPS, led to premature cell senescence in various cancer cells and in a xenograft tumor mouse model. Sodium chlorate, a metabolic inhibitor of HS sulfation also induced a cellular senescence phenotype. p53 and p21 accumulation was essential for PAPSS2-mediated cellular senescence. Such senescence phenotypes were closely correlated with cell surface HS levels in both cancer cells and human diploid fibroblasts. The determination of the activation of receptors such as FGFR1, Met, and insulin growth factor 1 receptor β indicated that the augmented FGFR1/AKT signaling was specifically involved in premature senescence in a HS-dependent manner. Thus, blockade of either FGFR1 or AKT prohibited p53 and p21 accumulation and cell fate switched from cellular senescence to apoptosis. In particular, desulfation at the 2-O position in the HS chain contributed to the premature senescence via the augmented FGFR1 signaling. Taken together, we reveal, for the first time, that the proper status of HS is essential for the prevention of cellular senescence. These observations allowed us to hypothesize that the FGF/FGFR signaling system could initiate novel tumor defenses through regulating premature senescence.
Figures




References
-
- Narita M, Lowe SW. Senescence comes of age. Nat Med 2005; 11: 920–922. - PubMed
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8: 729–740. - PubMed
-
- Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 2008; 20: 150–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

