Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers?
- PMID: 26251351
- PMCID: PMC4696584
- DOI: 10.1681/ASN.2015010074
Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers?
Abstract
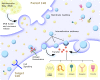
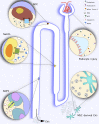
Extracellular vesicles from the urine and circulation have gained significant interest as potential diagnostic biomarkers in renal diseases. Urinary extracellular vesicles contain proteins from all sections of the nephron, whereas most studied circulating extracellular vesicles are derived from platelets, immune cells, and the endothelium. In addition to their diagnostic role as markers of kidney and vascular damage, extracellular vesicles may have functional significance in renal health and disease by facilitating communication between cells and protecting against kidney injury and bacterial infection in the urinary tract. However, the current understanding of extracellular vesicles has derived mostly from studies with very small numbers of patients or in vitro data. Moreover, accurate assessment of these vesicles remains a challenge, in part because of a lack of consensus in the methodologies to measure extracellular vesicles and the inability of most techniques to capture the entire size range of these vesicles. However, newer techniques and standardized protocols to improve the detection of extracellular vesicles are in development. A clearer understanding of the composition and biology of extracellular vesicles will provide insights into their pathophysiologic, diagnostic, and therapeutic roles.
Keywords: biomarker; exosomes; extracellular vesicles; kidney disease; microparticles.
Copyright © 2016 by the American Society of Nephrology.
Figures


References
-
- Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM: Microparticles: Biomarkers and beyond. Clin Sci (Lond) 124: 423–441, 2013 - PubMed
-
- Dear JW, Street JM, Bailey MA: Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 13: 1572–1580, 2013 - PubMed
-
- Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014 - PubMed
-
- Wolf P: The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

