Effect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite-host interaction
- PMID: 26251568
- PMCID: PMC4524381
- DOI: 10.2147/DDDT.S77860
Effect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite-host interaction
Erratum in
-
Erratum: Effect of Lactoferrin Protein on Red Blood Cells and Macrophages: Mechanism of Parasite-Host Interaction [Corrigendum].Drug Des Devel Ther. 2020 May 28;14:2123-2124. doi: 10.2147/DDDT.S258146. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32581511 Free PMC article.
Abstract
Background: Lactoferrin is a natural multifunctional protein known to have antitumor, antimicrobial, and anti-inflammatory activity. Apart from its antimicrobial effects, lactoferrin is known to boost the immune response by enhancing antioxidants. Lactoferrin exists in various forms depending on its iron saturation. The present study was done to observe the effect of lactoferrin, isolated from bovine and buffalo colostrum, on red blood cells (RBCs) and macrophages (human monocytic cell line-derived macrophages THP1 cells).
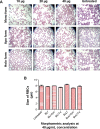
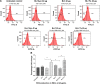
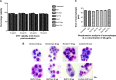
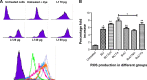
Methods: Lactoferrin obtained from both species and in different iron saturation forms were used in the present study, and treatment of host cells were given with different forms of lactoferrin at different concentrations. These treated host cells were used for various studies, including morphometric analysis, viability by MTT assay, survivin gene expression, production of reactive oxygen species, phagocytic properties, invasion assay, and Toll-like receptor-4, Toll-like receptor-9, and MDR1 expression, to investigate the interaction between lactoferrin and host cells and the possible mechanism of action with regard to parasitic infections.
Results: The mechanism of interaction between host cells and lactoferrin have shown various aspects of gene expression and cellular activity depending on the degree of iron saturation of lactoferrin. A significant increase (P<0.05) in production of reactive oxygen species, phagocytic activity, and Toll-like receptor expression was observed in host cells incubated with iron-saturated lactoferrin when compared with an untreated control group. However, there was no significant (P>0.05) change in percentage viability in the different groups of host cells treated, and no downregulation of survivin gene expression was found at 48 hours post-incubation. Upregulation of the Toll-like receptor and downregulation of the P-gp gene confirmed the immunomodulatory potential of lactoferrin protein.
Conclusion: The present study details the interaction between lactoferrin and parasite host cells, ie, RBCs and macrophages, using various cellular processes and expression studies. The study reveals the possible mechanism of action against various intracellular pathogens such as Toxoplasma, Plasmodium, Leishmania, Trypanosoma, and Mycobacterium. The presence of iron in lactoferrin plays an important role in enhancing the various activities taking place inside these cells. This work provides a lot of information about targeting lactoferrin against many parasitic infections which can rule out the exact pathways for inhibition of diseases caused by intracellular microbes mainly targeting RBCs and macrophages for their survival. Therefore, this initial study can serve as a baseline for further evaluation of the mechanism of action of lactoferrin against parasitic diseases, which is not fully understood to date.
Keywords: cytotoxicity; lactoferrin; morphometric analysis; phagocytosis.
Figures



References
-
- Metz-Boutigue MH, Jolles J, Mazurier J, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984;145(3):659–676. - PubMed
-
- Steijns JM, van Hooijdonk AC. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr. 2000;84(Suppl 1):S11–S17. - PubMed
-
- Sorensen M, Sorensen SPL. The proteins in whey. Comptes-Rendus des Travaux du Laboratoire Carlsberg. 1939;23:55–99.
-
- Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301.e301–e308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

