In vitro reversion of activated primary human hepatic stellate cells
- PMID: 26251672
- PMCID: PMC4527231
- DOI: 10.1186/s13069-015-0031-z
In vitro reversion of activated primary human hepatic stellate cells
Abstract
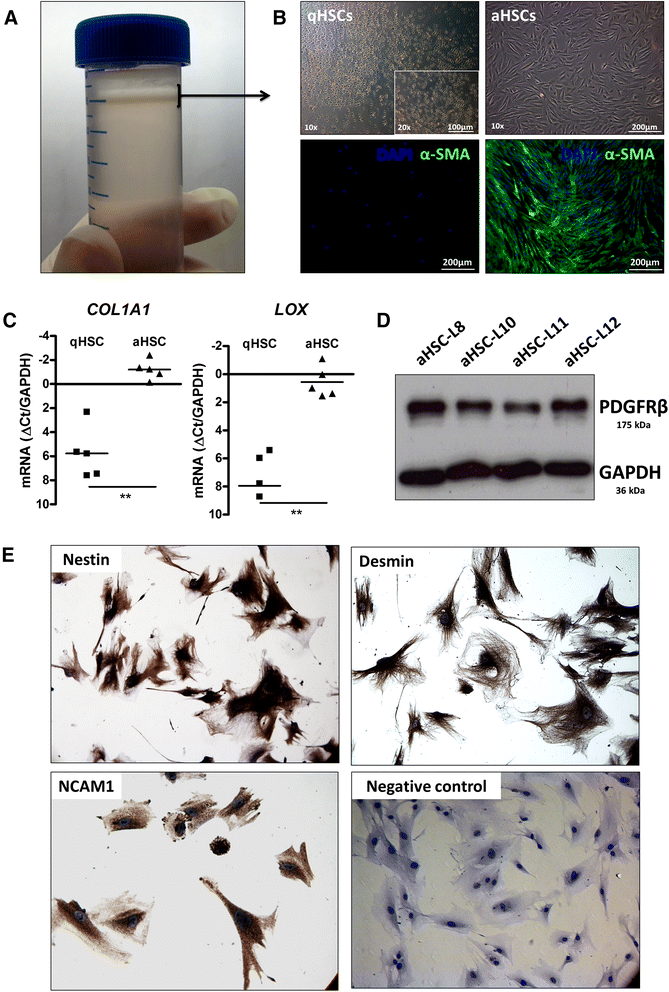
Background: Liver fibrosis is characterized by the excessive formation and accumulation of matrix proteins as a result of wound healing in the liver. A main event during fibrogenesis is the activation of the liver resident quiescent hepatic stellate cell (qHSC). Recent studies suggest that reversion of the activated HSC (aHSC) phenotype into a quiescent-like phenotype could be a major cellular mechanism underlying fibrosis regression in the liver, thereby offering new therapeutic perspectives for the treatment of liver fibrosis. Whether human HSCs have the ability to undergo a similar reversion in phenotype is currently unknown. The aim of the present study is to identify experimental conditions that can revert the in vitro activated phenotype of primary human HSCs and consequently to map the molecular events associated with this reversion process by gene expression profiling.
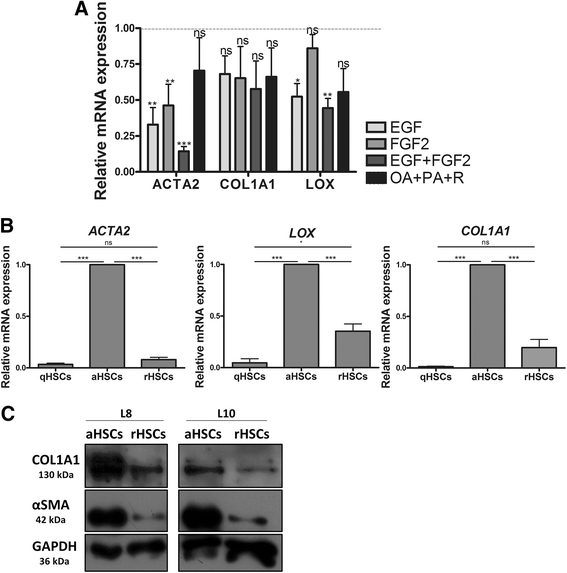
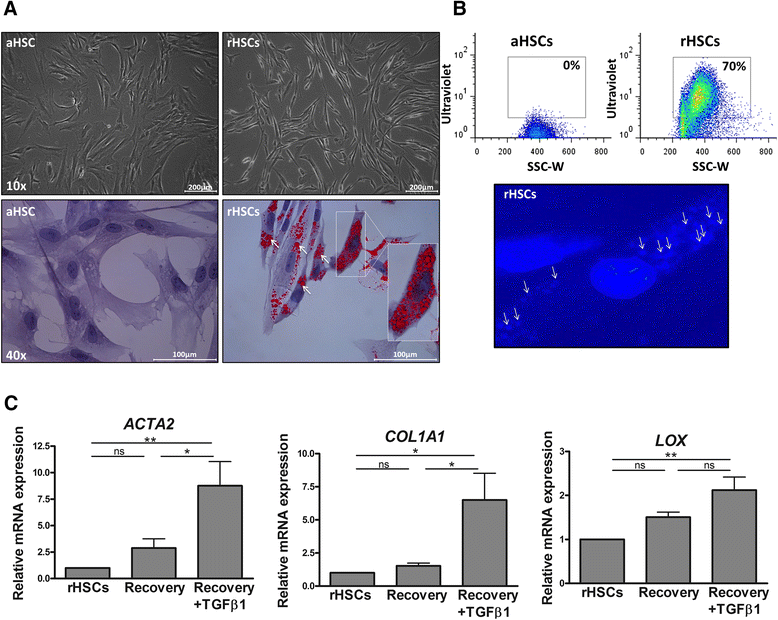
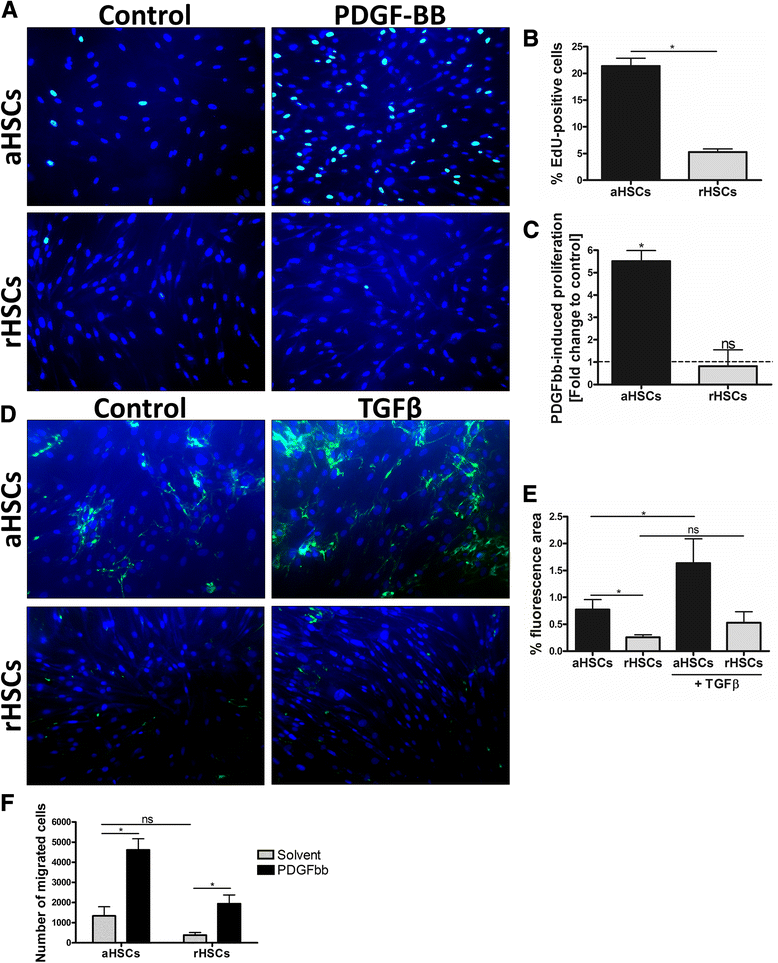
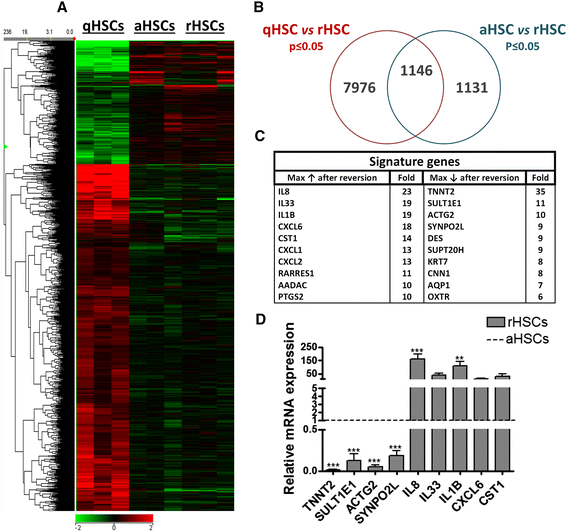
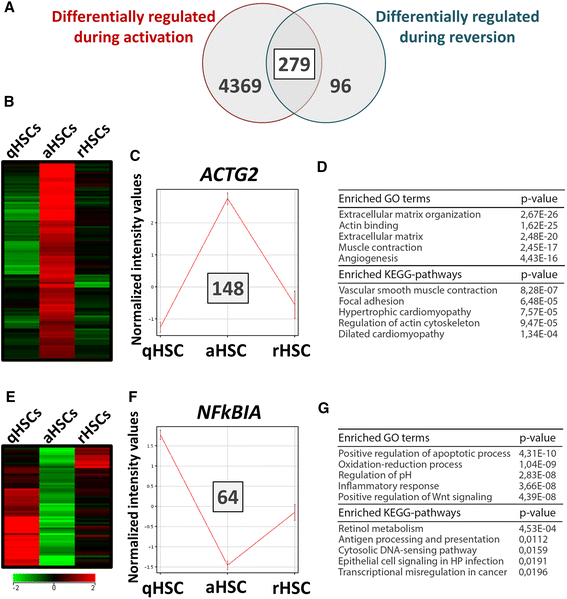
Results: We find that epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) synergistically downregulate the expression of ACTA2 and LOX in primary human aHSCs. Their combination with oleic acid, palmitic acid, and retinol further potentiates a more quiescent-like phenotype as demonstrated by the abundant presence of retinyl ester-positive intra-cytoplasmic lipid droplets, low expression levels of activation markers, and a reduced basal as well as cytokine-stimulated proliferation and matrix metalloproteinase activity. Gene expression profiling experiments reveal that these in vitro reverted primary human HSCs (rHSCs) display an intermediary phenotype that is distinct from qHSCs and aHSCs. Interestingly, this intermediary phenotype is characterized by the increased expression of several previously identified signature genes of in vivo inactivated mouse HSCs such as CXCL1, CXCL2, and CTSS, suggesting also a potential role for these genes in promoting a quiescent-like phenotype in human HSCs.
Conclusions: We provide evidence for the ability of human primary aHSCs to revert in vitro to a transitional state through synergistic action of EGF, FGF2, dietary fatty acids and retinol, and provide a first phenotypic and genomic characterization of human in vitro rHSCs.
Keywords: Fibrosis; Gene expression profiling; Hepatic stellate cells; Inactivation; Quiescent; Reversion.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

