G-Protein-Coupled Receptors: Next Generation Therapeutic Targets in Head and Neck Cancer?
- PMID: 26251921
- PMCID: PMC4549734
- DOI: 10.3390/toxins7082959
G-Protein-Coupled Receptors: Next Generation Therapeutic Targets in Head and Neck Cancer?
Abstract
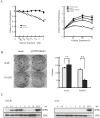
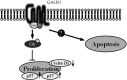
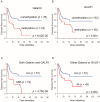
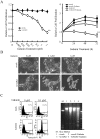
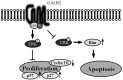
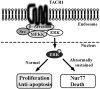
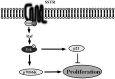
Therapeutic outcome in head and neck squamous cell carcinoma (HNSCC) is poor in most advanced cases. To improve therapeutic efficiency, novel therapeutic targets and prognostic factors must be discovered. Our studies have identified several G protein-coupled receptors (GPCRs) as promising candidates. Significant epigenetic silencing of GPCR expression occurs in HNSCC compared with normal tissue, and is significantly correlated with clinical behavior. Together with the finding that GPCR activity can suppress tumor cell growth, this indicates that GPCR expression has potential utility as a prognostic factor. In this review, we discuss the roles that galanin receptor type 1 (GALR1) and type 2 (GALR2), tachykinin receptor type 1 (TACR1), and somatostatin receptor type 1 (SST1) play in HNSCC. GALR1 inhibits proliferation of HNSCC cells though ERK1/2-mediated effects on cell cycle control proteins such as p27, p57, and cyclin D1, whereas GALR2 inhibits cell proliferation and induces apoptosis in HNSCC cells. Hypermethylation of GALR1, GALR2, TACR1, and SST1 is associated with significantly reduced disease-free survival and a higher recurrence rate. Although their overall activities varies, each of these GPCRs has value as both a prognostic factor and a therapeutic target. These data indicate that further study of GPCRs is a promising strategy that will enrich pharmacogenomics and prognostic research in HNSCC.
Keywords: biomarker; head and neck neoplasm; molecular targeted therapy; treatment.
Figures
Similar articles
-
Aberrant methylation inactivates somatostatin and somatostatin receptor type 1 in head and neck squamous cell carcinoma.PLoS One. 2015 Mar 3;10(3):e0118588. doi: 10.1371/journal.pone.0118588. eCollection 2015. PLoS One. 2015. PMID: 25734919 Free PMC article.
-
Galanin receptor subtypes 1 and 2 as therapeutic targets in head and neck squamous cell carcinoma.Expert Opin Ther Targets. 2010 Mar;14(3):289-302. doi: 10.1517/14728221003598922. Expert Opin Ther Targets. 2010. PMID: 20148716 Review.
-
Galanin receptor subtype 2 suppresses cell proliferation and induces apoptosis in p53 mutant head and neck cancer cells.Clin Cancer Res. 2009 Apr 1;15(7):2222-30. doi: 10.1158/1078-0432.CCR-08-2443. Epub 2009 Mar 10. Clin Cancer Res. 2009. PMID: 19276245 Free PMC article.
-
Galanin receptor 2 utilizes distinct signaling pathways to suppress cell proliferation and induce apoptosis in HNSCC.Mol Med Rep. 2014 Sep;10(3):1289-94. doi: 10.3892/mmr.2014.2362. Epub 2014 Jul 4. Mol Med Rep. 2014. PMID: 25017118
-
Promoter methylation of galanin receptors as epigenetic biomarkers for head and neck squamous cell carcinomas.Expert Rev Mol Diagn. 2019 Feb;19(2):137-148. doi: 10.1080/14737159.2019.1567334. Epub 2019 Jan 21. Expert Rev Mol Diagn. 2019. PMID: 30640567 Review.
Cited by
-
Prognostic value of aberrant promoter hypermethylation of tumor-related genes in early-stage head and neck cancer.Oncotarget. 2016 May 3;7(18):26087-98. doi: 10.18632/oncotarget.8317. Oncotarget. 2016. PMID: 27027429 Free PMC article.
-
HIF-1α and mTOR - Possible Novel Strategies of Targeted Therapies in p16-positive and -negative HNSCC.Cancer Genomics Proteomics. 2018 May-Jun;15(3):175-184. doi: 10.21873/cgp.20075. Cancer Genomics Proteomics. 2018. PMID: 29695399 Free PMC article.
-
miR-206 Promotes Cancer Progression by Targeting Full-Length Neurokinin-1 Receptor in Breast Cancer.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819875168. doi: 10.1177/1533033819875168. Technol Cancer Res Treat. 2019. PMID: 31506061 Free PMC article.
-
Genes encoding neuropeptide receptors are epigenetic markers in patients with head and neck cancer: a site-specific analysis.Oncotarget. 2017 Jul 18;8(44):76318-76328. doi: 10.18632/oncotarget.19356. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100314 Free PMC article.
-
Portrait of DNA methylated genes predictive of poor prognosis in head and neck cancer and the implication for targeted therapy.Sci Rep. 2021 May 11;11(1):10012. doi: 10.1038/s41598-021-89476-x. Sci Rep. 2021. PMID: 33976322 Free PMC article.
References
-
- Parfenov M., Pedamallu C.S., Gehlenborg N., Freeman S.S., Danilova L., Bristow C.A., Lee S., Hadjipanayis A.G., Ivanova E.V., Wilkerson M.D., et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl. Acad. Sci. USA. 2014;111:15544–15549. doi: 10.1073/pnas.1416074111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

