Use of a Novel Integrase-Deficient Lentivirus for Targeted Anti-Cancer Therapy With Survivin Promoter-Driven Diphtheria Toxin A
- PMID: 26252309
- PMCID: PMC4616595
- DOI: 10.1097/MD.0000000000001301
Use of a Novel Integrase-Deficient Lentivirus for Targeted Anti-Cancer Therapy With Survivin Promoter-Driven Diphtheria Toxin A
Abstract
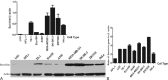
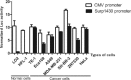
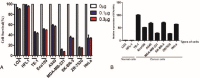
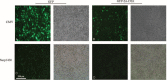
As an immunotoxin, diphtheria toxin has been widely used in gene therapy and gene function assays for its roles in protein synthesis inhibition, and the aim of our study is to set up a nonintegrating lentiviral system for specific expression of diphtheria toxin A (DTA) used in cancer gene therapy.Here, we established a lentiviral system that could coordinately express fluorescent protein and DTA driven by the cytomegalovirus (CMV) promoter, which is convenient for us to precisely trace the expression of DTA and monitor the process of lentivirus packaging. To achieve safer cancer therapy, we replaced the CMV promoter with the Survivin promoter, a specific promoter that is dramatically activated in cancer tissues and cells, but not in normal tissues and cells, and that will impose greater therapeutic potential because a significant expression difference occurred between these 2 groups. Meanwhile, we obtained integrase-deficient lentivirus (IDLV) after packaging with the integrase mutant, which expresses defective integrase RRK262263264AAH, to minimize the side effects that derived from the insertional mutagenesis of the host genome.Our results suggest that the IDLV system that we generated possesses therapeutic potential in cancers in vitro and in vivo.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Transient Expression of Green Fluorescent Protein in Integrase-Defective Lentiviral Vector-Transduced 293T Cell Line.Methods Mol Biol. 2016;1448:159-73. doi: 10.1007/978-1-4939-3753-0_12. Methods Mol Biol. 2016. PMID: 27317180
-
The therapeutic potential of attenuated diphtheria toxin delivered by an adenovirus vector with survivin promoter on human lung cancer cells.Cancer Biol Ther. 2021 Jan 2;22(1):79-87. doi: 10.1080/15384047.2020.1859870. Epub 2020 Dec 30. Cancer Biol Ther. 2021. PMID: 33377426 Free PMC article.
-
Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer.Cancer Res. 2002 May 1;62(9):2576-82. Cancer Res. 2002. PMID: 11980652
-
Integrase-deficient lentivirus: opportunities and challenges for human gene therapy.Curr Gene Ther. 2014;14(5):352-64. doi: 10.2174/1566523214666140825124311. Curr Gene Ther. 2014. PMID: 25174579 Review.
-
Integrase-defective lentiviral vectors--a stage for nonviral integration machineries.Curr Gene Ther. 2011 Oct;11(5):350-62. doi: 10.2174/156652311797415881. Curr Gene Ther. 2011. PMID: 21745178 Review.
Cited by
-
Diphtheria Toxin A-Resistant Cell Lines Enable Robust Production and Evaluation of DTA-Encoding Lentiviruses.Sci Rep. 2019 Jun 20;9(1):8985. doi: 10.1038/s41598-019-45481-9. Sci Rep. 2019. PMID: 31222087 Free PMC article.
-
The Old and the New: Prospects for Non-Integrating Lentiviral Vector Technology.Viruses. 2020 Sep 29;12(10):1103. doi: 10.3390/v12101103. Viruses. 2020. PMID: 33003492 Free PMC article. Review.
-
Gene Therapy for Liver Cancers: Current Status from Basic to Clinics.Cancers (Basel). 2019 Nov 25;11(12):1865. doi: 10.3390/cancers11121865. Cancers (Basel). 2019. PMID: 31769427 Free PMC article. Review.
-
Integrase deficient lentiviral vector: prospects for safe clinical applications.PeerJ. 2022 Aug 12;10:e13704. doi: 10.7717/peerj.13704. eCollection 2022. PeerJ. 2022. PMID: 35979475 Free PMC article.
-
Effect of Diphtheria Toxin-Based Gene Therapy for Hepatocellular Carcinoma.Cancers (Basel). 2020 Feb 18;12(2):472. doi: 10.3390/cancers12020472. Cancers (Basel). 2020. PMID: 32085552 Free PMC article.
References
-
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3:917–921. - PubMed
-
- Bao R, Connolly DC, Murphy M, et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst 2002; 94:522–528. - PubMed
-
- Chen JS, Liu JC, Shen L, et al. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer Gene Ther 2004; 11:740–747. - PubMed
-
- Tanaka K, Iwamoto S, Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res 2000; 6:127–134. - PubMed
-
- Zhang M, Zhang X, Zhao S, et al. Prognostic value of survivin and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Target Oncol 2014; 9:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

