Stimulation of Glia Reveals Modulation of Mammalian Spinal Motor Networks by Adenosine
- PMID: 26252389
- PMCID: PMC4529192
- DOI: 10.1371/journal.pone.0134488
Stimulation of Glia Reveals Modulation of Mammalian Spinal Motor Networks by Adenosine
Abstract
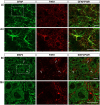
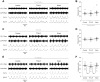
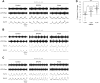
Despite considerable evidence that glia can release modulators to influence the excitability of neighbouring neurons, the importance of gliotransmission for the operation of neural networks and in shaping behaviour remains controversial. Here we characterise the contribution of glia to the modulation of the mammalian spinal central pattern generator for locomotion, the output of which is directly relatable to a defined behaviour. Glia were stimulated by specific activation of protease-activated receptor-1 (PAR1), an endogenous G-protein coupled receptor preferentially expressed by spinal glia during ongoing activity of the spinal central pattern generator for locomotion. Selective activation of PAR1 by the agonist TFLLR resulted in a reversible reduction in the frequency of locomotor-related bursting recorded from ventral roots of spinal cord preparations isolated from neonatal mice. In the presence of the gliotoxins methionine sulfoximine or fluoroacetate, TFLLR had no effect, confirming the specificity of PAR1 activation to glia. The modulation of burst frequency upon PAR1 activation was blocked by the non-selective adenosine-receptor antagonist theophylline and by the A1-receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine, but not by the A2A-receptor antagonist SCH5826, indicating production of extracellular adenosine upon glial stimulation, followed by A1-receptor mediated inhibition of neuronal activity. Modulation of network output following glial stimulation was also blocked by the ectonucleotidase inhibitor ARL67156, indicating glial release of ATP and its subsequent degradation to adenosine rather than direct release of adenosine. Glial stimulation had no effect on rhythmic activity recorded following blockade of inhibitory transmission, suggesting that glial cell-derived adenosine acts via inhibitory circuit components to modulate locomotor-related output. Finally, the modulation of network output by endogenous adenosine was found to scale with the frequency of network activity, implying activity-dependent release of adenosine. Together, these data indicate that glia play an active role in the modulation of mammalian locomotor networks, providing negative feedback control that may stabilise network activity.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

