Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in vivo Osteochondral Defect Model
- PMID: 26252391
- PMCID: PMC4529143
- DOI: 10.1371/journal.pone.0133937
Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in vivo Osteochondral Defect Model
Abstract
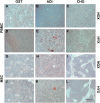
This study characterized peripheral blood mononuclear cells (PBMC) in terms of their potential in cartilage repair and investigated their ability to improve the healing in a pre-clinical large animal model. Human PBMCs were isolated with gradient centrifugation and adherent PBMC's were evaluated for their ability to differentiate into adipogenic, chondrogenic and osteogenic lineages and also for their expression of musculoskeletal genes. The phenotype of the PBMCs was evaluated using Stro-1, CD34, CD44, CD45, CD90, CD106, CD105, CD146 and CD166 cell surface markers. Osteochondral defects were created in the medial femoral condyle (MFC) of 24 Welsh mountain sheep and evaluated at a six month time point. Four cell treatment groups were evaluated in combination with collagen-GAG-scaffold: (1) MSC alone; (2) MSCs and PBMCs at a ratio of 20:1; (3) MSCs and PBMC at a ratio of 2:1 and (4) PBMCs alone. Samples from the surgical site were evaluated for mechanical properties, ICRS score and histological repair. Fresh PBMC samples were 90% positive for hematopoietic cell surface markers and negative for the MSC antibody panel (<1%, p = 0.006). However, the adherent PBMC population expressed mesenchymal stem cell markers in hypoxic culture and lacked CD34/45 positive cells (<0.2%). This finding demonstrated that the adherent cells had acquired an MSC-like phenotype and transformed in hypoxia from their original hematopoietic lineage. Four key genes in muskuloskeletal biology were significantly upregulated in adherent PBMCs by hypoxia: BMP2 4.2-fold (p = 0.0007), BMP6 10.7-fold (p = 0.0004), GDF5 2.0-fold (p = 0.002) and COL1 5.0-fold (p = 0.046). The monolayer multilineage analysis confirmed the trilineage mesenchymal potential of the adherent PBMCs. PBMC cell therapy was equally good as bone marrow MSC therapy for defects in the ovine large animal model. Our results show that PBMCs support cartilage healing and oxygen tension of the environment was found to have a key effect on the derivation of a novel adherent cell population with an MSC-like phenotype. This study presents a novel and easily attainable point-of-care cell therapy with PBMCs to treat osteochondral defects in the knee avoiding any cell manipulations outside the surgical room.
Conflict of interest statement
Figures




Comment in
-
Regenerative medicine. PBMCs stimulate chondrocyte migration and cartilage repair.Nat Rev Rheumatol. 2015 Oct;11(10):563. doi: 10.1038/nrrheum.2015.118. Epub 2015 Aug 25. Nat Rev Rheumatol. 2015. PMID: 26303955 No abstract available.
References
-
- Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects. An experimental study in horses. Clinical orthopaedics and related research. 1972;82:253–62. . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

