Lamprey VLRB response to influenza virus supports universal rules of immunogenicity and antigenicity
- PMID: 26252514
- PMCID: PMC4552221
- DOI: 10.7554/eLife.07467
Lamprey VLRB response to influenza virus supports universal rules of immunogenicity and antigenicity
Abstract
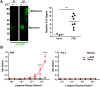
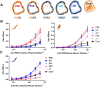
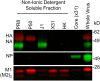
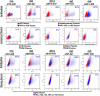
Immunoglobulins (Igs) are a crown jewel of jawed vertebrate evolution. Through recombination and mutation of small numbers of genes, Igs can specifically recognize a vast variety of natural and man-made organic molecules. Jawless vertebrates evolved a parallel system of humoral immunity, which recognizes antigens not with Ig, but with a structurally unrelated receptor called the variable lymphocyte receptor B (VLRB). We exploited the convergent evolution of Ig and VLRB antibodies (Abs) to investigate if intrinsic chemical features of foreign proteins determine their antigenicity and immunogenicity. Surprisingly, we find lamprey VLRB and mouse Ig responses to influenza A virus are extremely similar. Each focuses ~80% of the response on hemagglutinin (HA), mainly through recognition of the major antigenic sites in the HA globular head domain. Our findings predict basic conservation of Ab responses to protein antigens, strongly supporting the use of animal models for understanding human Ab responses to viruses and protein immunogens.
Keywords: antibodies; antigenicity; hemagglutinin; immunodominance; immunology; infectious disease; influenza; lamprey; microbiology; mouse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




References
-
- Castro R, Jouneau L, Pham HP, Bouchez O, Giudicelli V, Lefranc MP, Quillet E, Benmansour A, Cazals F, Six A, Fillatreau S, Sunyer O, Boudinot P. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLOS Pathogens. 2013;9:e1003098. doi: 10.1371/journal.ppat.1003098. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

