Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease
- PMID: 26252777
- PMCID: PMC4529214
- DOI: 10.1371/journal.pone.0134172
Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
Objectives: A randomized, parallel controlled, open-label clinical trial was conducted to evaluate the effect of a botanic compound berberine (BBR) on NAFLD.
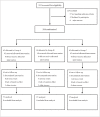
Methods: A randomized, parallel controlled, open-label clinical trial was conducted in three medical centers (NIH Registration number: NCT00633282). A total of 184 eligible patients with NAFLD were enrolled and randomly received (i) lifestyle intervention (LSI), (ii) LSI plus pioglitazone (PGZ) 15mg qd, and (iii) LSI plus BBR 0.5g tid, respectively, for 16 weeks. Hepatic fat content (HFC), serum glucose and lipid profiles, liver enzymes and serum and urine BBR concentrations were assessed before and after treatment. We also analyzed hepatic BBR content and expression of genes related to glucose and lipid metabolism in an animal model of NAFLD treated with BBR.
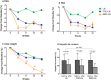
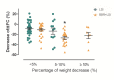
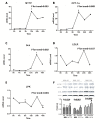
Results: As compared with LSI, BBR treatment plus LSI resulted in a significant reduction of HFC (52.7% vs 36.4%, p = 0.008), paralleled with better improvement in body weight, HOMA-IR, and serum lipid profiles (all p<0.05). BBR was more effective than PGZ 15mg qd in reducing body weight and improving lipid profile. BBR-related adverse events were mild and mainly occurred in digestive system. Serum and urine BBR concentrations were 6.99ng/ml and 79.2ng/ml, respectively, in the BBR-treated subjects. Animal experiments showed that BBR located favorably in the liver and altered hepatic metabolism-related gene expression.
Conclusion: BBR ameliorates NAFLD and related metabolic disorders. The therapeutic effect of BBR on NAFLD may involve a direct regulation of hepatic lipid metabolism.
Trial registration: ClinicalTrials.gov NCT00633282.
Conflict of interest statement
Figures

References
-
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. - PubMed
-
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

