Human respiratory syncytial virus non-structural protein NS1 modifies miR-24 expression via transforming growth factor-β
- PMID: 26253191
- PMCID: PMC4806578
- DOI: 10.1099/jgv.0.000261
Human respiratory syncytial virus non-structural protein NS1 modifies miR-24 expression via transforming growth factor-β
Abstract
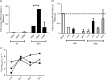
Human respiratory syncytial virus (RSV) is a major health challenge in the young and elderly owing to the lack of a safe and effective vaccine and proven antiviral drugs. Understanding the mechanisms by which viral genes and proteins modulate the host response to infection is critical for identifying novel disease intervention strategies. In this study, the RSV non-structural protein NS1 was shown to suppress miR-24 expression during infection. Lack of NS1 was linked to increased expression of miR-24, whilst NS1 overexpression suppressed miR-24 expression. NS1 was found to induce Kruppel-like factor 6 (KLF6), a transcription factor that positively regulates the transforming growth factor (TGF)-b pathway to induce cell cycle arrest. Silencing of KLF6 led to increased miR-24 expression via downregulation of TGF-β. Treatment with exogenous TGF-β suppressed miR-24 expression and induced KLF6. Confocal microscopy showed co-localization of KLF6 and RSV NS1. These findings indicated that RSV NS1 interacts with KLF6 and modulates miR-24 expression and TGF-β, which facilitates RSV replication.
Figures



Similar articles
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
-
Krüppel-like factor 6 regulates transforming growth factor-β gene expression during human respiratory syncytial virus infection.Virol J. 2011 Aug 17;8:409. doi: 10.1186/1743-422X-8-409. Virol J. 2011. PMID: 21849067 Free PMC article.
-
Respiratory syncytial virus non-structural protein 1 facilitates virus replication through miR-29a-mediated inhibition of interferon-α receptor.Biochem Biophys Res Commun. 2016 Sep 23;478(3):1436-41. doi: 10.1016/j.bbrc.2016.08.142. Epub 2016 Aug 25. Biochem Biophys Res Commun. 2016. PMID: 27569280
-
Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription.Cell Rep. 2021 Oct 12;37(2):109803. doi: 10.1016/j.celrep.2021.109803. Cell Rep. 2021. PMID: 34644581 Free PMC article.
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
Cited by
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
-
MicroRNAs in Asthma and Respiratory Infections: Identifying Common Pathways.Allergy Asthma Immunol Res. 2020 Jan;12(1):4-23. doi: 10.4168/aair.2020.12.1.4. Allergy Asthma Immunol Res. 2020. PMID: 31743961 Free PMC article. Review.
-
Autophagy, TGF-β, and SMAD-2/3 Signaling Regulates Interferon-β Response in Respiratory Syncytial Virus Infected Macrophages.Front Cell Infect Microbiol. 2016 Dec 12;6:174. doi: 10.3389/fcimb.2016.00174. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 28018859 Free PMC article.
-
Peripheral blood microRNAs expression is associated with infant respiratory syncytial virus infection.Oncotarget. 2017 Jul 18;8(57):96627-96635. doi: 10.18632/oncotarget.19364. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228557 Free PMC article.
-
Current State and Challenges in Developing Respiratory Syncytial Virus Vaccines.Vaccines (Basel). 2020 Nov 11;8(4):672. doi: 10.3390/vaccines8040672. Vaccines (Basel). 2020. PMID: 33187337 Free PMC article. Review.
References
-
- Basak A., Zhong M., Munzer J.S., Chrétien M., Seidah N.G. (2001). Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses: a comparative analysis with fluorogenic peptides Biochem J 353 537–545 10.1042/0264-6021:3530537 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

