Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo
- PMID: 26253611
- PMCID: PMC4613970
- DOI: 10.2337/db15-0244
Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo
Abstract
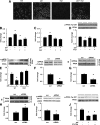
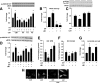
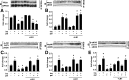
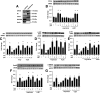
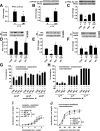
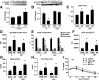
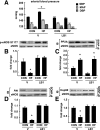
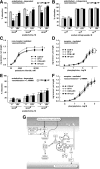
Prior studies have implicated accumulation of ceramide in blood vessels as a basis for vascular dysfunction in diet-induced obesity via a mechanism involving type 2 protein phosphatase (PP2A) dephosphorylation of endothelial nitric oxide synthase (eNOS). The current study sought to elucidate the mechanisms linking ceramide accumulation with PP2A activation and determine whether pharmacological inhibition of PP2A in vivo normalizes obesity-associated vascular dysfunction and limits the severity of hypertension. We show in endothelial cells that ceramide associates with the inhibitor 2 of PP2A (I2PP2A) in the cytosol, which disrupts the association of I2PP2A with PP2A leading to its translocation to the plasma membrane. The increased association between PP2A and eNOS at the plasma membrane promotes dissociation of an Akt-Hsp90-eNOS complex that is required for eNOS phosphorylation and activation. A novel small-molecule inhibitor of PP2A attenuated PP2A activation, prevented disruption of the Akt-Hsp90-eNOS complex in the vasculature, preserved arterial function, and maintained normal blood pressure in obese mice. These findings reveal a novel mechanism whereby ceramide initiates PP2A colocalization with eNOS and demonstrate that PP2A activation precipitates vascular dysfunction in diet-induced obesity. Therapeutic strategies targeted to reducing PP2A activation might be beneficial in attenuating vascular complications that exist in the context of type 2 diabetes, obesity, and conditions associated with insulin resistance.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
References
-
- Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens 2010;4:102–115 - PubMed
-
- Tripathy D, Mohanty P, Dhindsa S, et al. . Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003;52:2882–2887 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

