CXCL12 Gene Therapy Ameliorates Ischemia-Induced White Matter Injury in Mouse Brain
- PMID: 26253714
- PMCID: PMC4572904
- DOI: 10.5966/sctm.2015-0074
CXCL12 Gene Therapy Ameliorates Ischemia-Induced White Matter Injury in Mouse Brain
Abstract
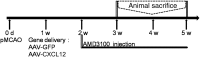
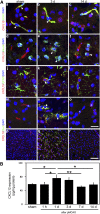
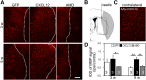
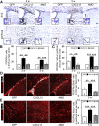
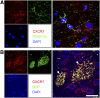
Remyelination is an important repair process after ischemic stroke-induced white matter injury. It often fails because of the insufficient recruitment of oligodendrocyte progenitor cells (OPCs) to the demyelinated site or the inefficient differentiation of OPCs to oligodendrocytes. We investigated whether CXCL12 gene therapy promoted remyelination after middle cerebral artery occlusion in adult mice. The results showed that CXCL12 gene therapy at 1 week after ischemia could protect myelin sheath integrity in the perifocal region, increase the number of platelet-derived growth factor receptor-α (PDGFRα)-positive and PDGFRα/bromodeoxyuridine-double positive OPCs in the subventricular zone, and further enhance their migration to the ischemic lesion area. Coadministration of AMD3100, the antagonist for CXCL12 receptor CXCR4, eliminated the beneficial effect of CXCL12 on myelin sheath integrity and negatively influenced OPC proliferation and migration. At 5 weeks after ischemia, CXCR4 was found on the PDGFRα- and/or neuron/glia type 2 (NG2)-positive OPCs but not on the myelin basic protein-positive mature myelin sheaths, and CXCR7 was only expressed on the mature myelin sheath in the ischemic mouse brain. Our data indicated that CXCL12 gene therapy effectively protected white matter and promoted its repair after ischemic injury. The treatment at 1 week after ischemia is effective, suggesting that this strategy has a longer therapeutic time window than the treatments currently available.
Significance: This study has demonstrated for the first time that CXCL12 gene therapy significantly ameliorates brain ischemia-induced white matter injury and promotes oligodendrocyte progenitor cell proliferation in the subventricular zone and migration to the perifocal area in the ischemic mouse brain. Additional data showed that CXCR4 receptor plays an important role during the proliferation and migration of oligodendrocyte progenitor cells, and CXCR7 might play a role during maturation. In contrast to many experimental studies that provide treatment before ischemic insult, CXCL12 gene therapy was performed 1 week after brain ischemia, which significantly prolonged the therapeutic time window of brain ischemia.
Keywords: C-X-C chemokine ligand 12; Ischemia; Oligodendrocyte progenitor cell; Remyelination; White matter.
©AlphaMed Press.
Figures

References
-
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. - PubMed
-
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. - PubMed
-
- Fork M, Bartels C, Ebert AD, et al. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj. 2005;19:101–108. - PubMed
-
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

