Evidence of the Primary Afferent Tracts Undergoing Neurodegeneration in Horses With Equine Degenerative Myeloencephalopathy Based on Calretinin Immunohistochemical Localization
- PMID: 26253880
- PMCID: PMC4831571
- DOI: 10.1177/0300985815598787
Evidence of the Primary Afferent Tracts Undergoing Neurodegeneration in Horses With Equine Degenerative Myeloencephalopathy Based on Calretinin Immunohistochemical Localization
Abstract
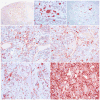
Equine degenerative myeloencephalopathy (EDM) is characterized by a symmetric general proprioceptive ataxia in young horses, and is likely underdiagnosed for 2 reasons: first, clinical signs overlap those of cervical vertebral compressive myelopathy; second, histologic lesions--including axonal spheroids in specific tracts of the somatosensory and motor systems--may be subtle. The purpose of this study was (1) to utilize immunohistochemical (IHC) markers to trace axons in the spinocuneocerebellar, dorsal column-medial lemniscal, and dorsospinocerebellar tracts in healthy horses and (2) to determine the IHC staining characteristics of the neurons and degenerated axons along the somatosensory tracts in EDM-affected horses. Examination of brain, spinal cord, and nerves was performed on 2 age-matched control horses, 3 EDM-affected horses, and 2 age-matched disease-control horses via IHC for calbindin, vesicular glutamate transporter 2, parvalbumin, calretinin, glutamic acid decarboxylase, and glial fibrillary acidic protein. Primary afferent axons of the spinocuneocerebellar, dorsal column-medial lemniscal, and dorsospinocerebellar tracts were successfully traced with calretinin. Calretinin-positive cell bodies were identified in a subset of neurons in the dorsal root ganglia, suggesting that calretinin IHC could be used to trace axonal projections from these cell bodies. Calretinin-immunoreactive spheroids were present in EDM-affected horses within the nuclei cuneatus medialis, cuneatus lateralis, and thoracicus. Neurons within those nuclei were calretinin negative. Cell bodies of degenerated axons in EDM-affected horses are likely located in the dorsal root ganglia. These findings support the role of sensory axonal degeneration in the pathogenesis of EDM and provide a method to highlight tracts with axonal spheroids to aid in the diagnosis of this neurodegenerative disease.
Keywords: ataxia; calcium-binding proteins; horses; medulla oblongata neuroaxonal dystrophies; spinal cord.
© The Author(s) 2015.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Aleman M, Finno CJ, Higgins RJ, et al. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. J Am Vet Med Assoc. 2011;239(6):823–833. - PubMed
-
- Beech J. Neuroaxonal dystrophy of the accessory cuneate nucleus in horses. Vet Pathol. 1984;21(4):384–393. - PubMed
-
- Finno CJ, Higgins RJ, Aleman M, et al. Equine degenerative myeloencephalopathy in Lusitano horses. J Vet Intern Med. 2011;25(6):1439–1446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

