Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: a multidisciplinary study of peritoneal carcinomatosis
- PMID: 26254295
- PMCID: PMC4673199
- DOI: 10.18632/oncotarget.4309
Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: a multidisciplinary study of peritoneal carcinomatosis
Abstract
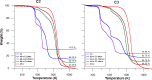
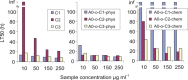
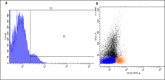
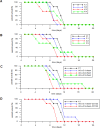
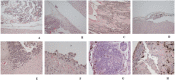
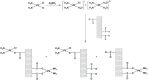
In general, detection of peritoneal carcinomatosis (PC) occurs at the late stage when there is no treatment option. In the present study, we designed novel drug delivery systems that are functionalized with anti-CD133 antibodies. The C1, C2 and C3 complexes with cisplatin were introduced into nanotubes, either physically or chemically. The complexes were reacted with anti-CD133 antibody to form the labeled product of A0-o-CX-chem-CD133. Cytotoxicity screening of all the complexes was performed on CHO cells. Data showed that both C2 and C3 Pt-complexes are more cytotoxic than C1. Flow-cytometry analysis showed that nanotubes conjugated to CD133 antibody have the ability to target cells expressing the CD133 antigen which is responsible for the emergence of resistance to chemotherapy and disease recurrence. The shortest survival rate was observed in the control mice group (K3) where no hyperthermic intraperitoneal chemotherapy procedures were used. On the other hand, the longest median survival rate was observed in the group treated with A0-o-C1-chem-CD133. In summary, we designed a novel drug delivery system based on carbon nanotubes loaded with Pt-prodrugs and functionalized with anti-CD133 antibodies. Our data demonstrates the effectiveness of the new drug delivery system and provides a novel therapeutic modality in the treatment of melanoma.
Keywords: carcinomatosis; hyperthermic intraperitoneal chemotherapy; intraperitoneal perfusion and nanovehicles; palliative.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



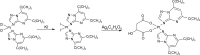


References
-
- Pilati P, Rossi CR, Mocellin S, Foletto M, Scagnet B, Pasetto L, Lise M. Multimodal treatment of peritoneal carcinomatosis and sarcomatosis. European Journal of Surgical Oncology. 2001;27:125–134. - PubMed
-
- Tsilimparis N, Bockelmann C, Raue W, Menenakos C, Perez S, Rau B, Hartmann J. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Annals of Surgical Oncology. 2013;20:226–232. - PubMed
-
- Bellavance EC, Alexander HR. Palliative interventions in patients with peritoneal metastases and malignant bowel obstruction. Journal of Clinical Oncology. 2012;30:4290–4291. - PubMed
-
- Roviello F, Caruso S, Neri A, Marrelli D. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. European Journal of Surgical Oncology. 2013;39:1309–1316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

