How do treadmill speed and terrain visibility influence neuromuscular control of guinea fowl locomotion?
- PMID: 26254324
- PMCID: PMC4631773
- DOI: 10.1242/jeb.104646
How do treadmill speed and terrain visibility influence neuromuscular control of guinea fowl locomotion?
Abstract
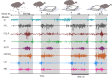
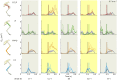
Locomotor control mechanisms must flexibly adapt to both anticipated and unexpected terrain changes to maintain movement and avoid a fall. Recent studies revealed that ground birds alter movement in advance of overground obstacles, but not treadmill obstacles, suggesting context-dependent shifts in the use of anticipatory control. We hypothesized that differences between overground and treadmill obstacle negotiation relate to differences in visual sensory information, which influence the ability to execute anticipatory manoeuvres. We explored two possible explanations: (1) previous treadmill obstacles may have been visually imperceptible, as they were low contrast to the tread, and (2) treadmill obstacles are visible for a shorter time compared with runway obstacles, limiting time available for visuomotor adjustments. To investigate these factors, we measured electromyographic activity in eight hindlimb muscles of the guinea fowl (Numida meleagris, N=6) during treadmill locomotion at two speeds (0.7 and 1.3 m s(-1)) and three terrain conditions at each speed: (i) level, (ii) repeated 5 cm low-contrast obstacles (<10% contrast, black/black), and (iii) repeated 5 cm high-contrast obstacles (>90% contrast, black/white). We hypothesized that anticipatory changes in muscle activity would be higher for (1) high-contrast obstacles and (2) the slower treadmill speed, when obstacle viewing time is longer. We found that treadmill speed significantly influenced obstacle negotiation strategy, but obstacle contrast did not. At the slower speed, we observed earlier and larger anticipatory increases in muscle activity and shifts in kinematic timing. We discuss possible visuomotor explanations for the observed context-dependent use of anticipatory strategies.
Keywords: Bipedal; Bird; Muscle; Numida meleagris; Stability; Visuomotor control.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures







References
-
- Akaike H. (1976). An information criterion (AIC). Math. Sci. 14, 5-9.
-
- Birn-Jeffery A. V. (2012). Scaling of running stability and limb posture with body size in galliform birds. PhD Thesis, Royal Veterinary College, UK.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

