ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity
- PMID: 26254336
- PMCID: PMC4666964
- DOI: 10.1152/ajpheart.00517.2014
ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity
Abstract
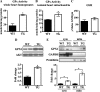
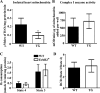
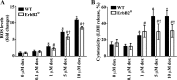
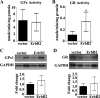
Levels of the HER2/ErbB2 protein in the heart are upregulated in some women during breast cancer therapy, and these women are at high risk for developing heart dysfunction after sequential treatment with anti-ErbB2/trastuzumab or doxorubicin. Doxorubicin is known to increase oxidative stress in the heart, and thus we considered the possibility that ErbB2 protein influences the status of cardiac antioxidant defenses in cardiomyocytes. In this study, we measured reactive oxygen species (ROS) in cardiac mitochondria and whole hearts from mice with cardiac-specific overexpression of ErbB2 (ErbB2(tg)) and found that, compared with control mice, high levels of ErbB2 in myocardium result in lower levels of ROS in mitochondria (P = 0.0075) and whole hearts (P = 0.0381). Neonatal cardiomyocytes isolated from ErbB2(tg) hearts have lower ROS levels and less cellular death (P < 0.0001) following doxorubicin treatment. Analyzing antioxidant enzyme levels and activities, we found that ErbB2(tg) hearts have increased levels of glutathione peroxidase 1 (GPx1) protein (P < 0.0001) and GPx activity (P = 0.0031) in addition to increased levels of two known GPx activators, c-Abl (P = 0.0284) and Arg (P < 0.0001). Interestingly, although mitochondrial ROS emission is reduced in the ErbB2(tg) hearts, oxygen consumption rates and complex I activity are similar to control littermates. Compared with these in vivo studies, H9c2 cells transfected with ErbB2 showed less cellular toxicity and produced less ROS (P < 0.0001) after doxorubicin treatment but upregulated GR activity (P = 0.0237) instead of GPx. Our study shows that ErbB2-dependent signaling contributes to antioxidant defenses and suggests a novel mechanism by which anticancer therapies involving ErbB2 antagonists can harm myocardial structure and function.
Keywords: ErbB2; glutathione peroxidase; mitochondria; nRTK; reactive oxygen species.
Copyright © 2015 the American Physiological Society.
Figures
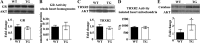



Comment in
-
Can ErbB2 overexpression protect against doxorubicin cardiotoxicity?Am J Physiol Heart Circ Physiol. 2015 Oct;309(8):H1235-6. doi: 10.1152/ajpheart.00647.2015. Epub 2015 Aug 21. Am J Physiol Heart Circ Physiol. 2015. PMID: 26297228 Free PMC article. No abstract available.
-
Letter to the editor: "Doxorubicin and ErbB2 overexpression: another piece in the mitochondrial jigsaw".Am J Physiol Heart Circ Physiol. 2016 May 1;310(9):H1275-6. doi: 10.1152/ajpheart.00179.2016. Am J Physiol Heart Circ Physiol. 2016. PMID: 27199386 No abstract available.
References
-
- Behr TM, Behe M, Wormann B. Trastuzumab and breast cancer. N Engl J Med 345: 995–996, 2001. - PubMed
-
- Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18: 847–855, 1997. - PubMed
-
- Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278: 39609–39614, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

