Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo
- PMID: 26254600
- PMCID: PMC4641790
- DOI: 10.1016/j.ydbio.2015.08.004
Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo
Abstract
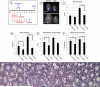
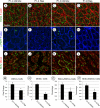
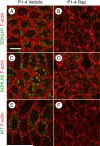
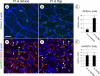
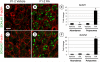
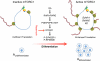
Spermatogonial stem cells (SSCs) must balance self-renewal with production of transit-amplifying progenitors that differentiate in response to retinoic acid (RA) before entering meiosis. This self-renewal vs. differentiation spermatogonial fate decision is critical for maintaining tissue homeostasis, as imbalances cause spermatogenesis defects that can lead to human testicular cancer or infertility. A great deal of effort has been exerted to understand how the SSC population is maintained. In contrast, little is known about the essential program of differentiation initiated by retinoic acid (RA) that precedes meiosis, and the pathways and proteins involved are poorly defined. We recently reported a novel role for RA in stimulating the PI3/AKT/mTOR kinase signaling pathway to activate translation of repressed mRNAs such as Kit. Here, we examined the requirement for mTOR complex 1 (mTORC1) in mediating the RA signal to direct spermatogonial differentiation in the neonatal testis. We found that in vivo inhibition of mTORC1 by rapamycin blocked spermatogonial differentiation, which led to an accumulation of undifferentiated spermatogonia. In addition, rapamycin also blocked the RA-induced translational activation of mRNAs encoding KIT, SOHLH1, and SOHLH2 without affecting expression of STRA8. These findings highlight dual roles for RA in germ cell development - transcriptional activation of genes, and kinase signaling to stimulate translation of repressed messages required for spermatogonial differentiation.
Keywords: Retinoic acid; Spermatogenesis; Spermatogonia; Testis; Translation; mTOR.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

