Antenatal Magnesium and Cerebral Palsy in Preterm Infants
- PMID: 26254839
- PMCID: PMC4587284
- DOI: 10.1016/j.jpeds.2015.06.067
Antenatal Magnesium and Cerebral Palsy in Preterm Infants
Abstract
Objective: To evaluate the relationship of maternal antenatal magnesium sulfate (MgSO4) with neonatal cranial ultrasound abnormalities and cerebral palsy (CP).
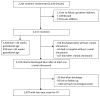
Study design: In a randomized trial of MgSO4 or placebo in women at high risk of preterm delivery, up to 3 cranial ultrasounds were obtained in the neonatal period. Images were reviewed by at least 2 pediatric radiologists masked to treatment and other clinical conditions. Diagnoses were predefined for intraventricular hemorrhage, periventricular leukomalacia, intracerebral echolucency or echodensity, and ventriculomegaly. CP was diagnosed at 2 years of age by standardized neurologic examination.
Results: Intraventricular hemorrhage, periventricular leukomalacia, intracerebral echolucency or echodensity, and ventriculomegaly were all strongly associated with an increased risk of CP. MgSO4 administration did not affect the risk of cranial ultrasound abnormality observed at 35 weeks postmenstrual age or later. However, for the 82% of infants born at <32 weeks gestation, MgSO4 was associated with a reduction in risk of echolucency or echodensity. The reduction in risk for echolucency explained 21% of the effect of MgSO4 on CP (P = .04), and for echodensity explained 20% of the effect (P = .02).
Conclusions: MgSO4 given prior to preterm delivery was associated with decreased risk of developing echodensities and echolucencies at <32 weeks gestation. However, this effect can only partially explain the effect of MgSO4 on CP at 2 years of age.
Trial registration: ClinicalTrials.gov: NCT00014989.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
References
-
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–68. - PubMed
-
- Jonsdottir GM, Georgsdottir I, Haraldsson A, Hardardottir H, Thorkelsson T, Dagbjartsson A. Survival and neurodevelopmental outcome of ELBW children at 5 years of age: comparison of two cohorts born 10 years apart. Acta Paediatr. 2012;101:714–718. - PubMed
-
- Latal B. Prediction of neurodevelopmental outcome after preterm birth. Pediatr Neurol. 2009;40:413–419. - PubMed
-
- Marlow N, Wolke D, Bracewell MA, Samara M EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD019897/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- HD27861/HD/NICHD NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- Z99 NS999999/ImNIH/Intramural NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD21414/HD/NICHD NIH HHS/United States
- U10 HD027905/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- M01-RR-000080/RR/NCRR NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD53907/HD/NICHD NIH HHS/United States
- HD34210/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD034122/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous