Cardiovascular magnetic resonance phase contrast imaging
- PMID: 26254979
- PMCID: PMC4529988
- DOI: 10.1186/s12968-015-0172-7
Cardiovascular magnetic resonance phase contrast imaging
Abstract
Cardiovascular magnetic resonance (CMR) phase contrast imaging has undergone a wide range of changes with the development and availability of improved calibration procedures, visualization tools, and analysis methods. This article provides a comprehensive review of the current state-of-the-art in CMR phase contrast imaging methodology, clinical applications including summaries of past clinical performance, and emerging research and clinical applications that utilize today's latest technology.
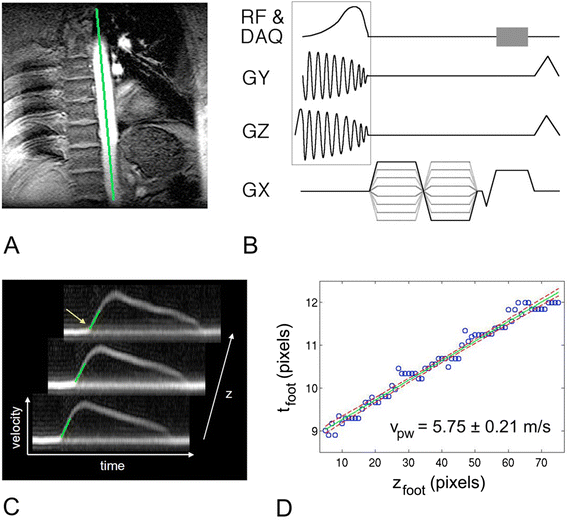
Figures
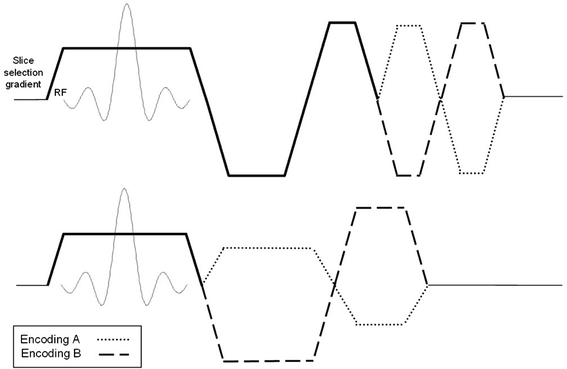
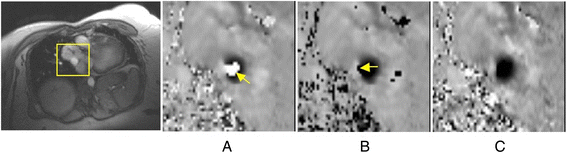
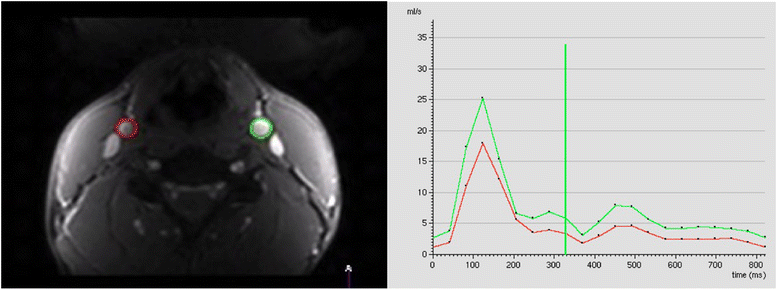
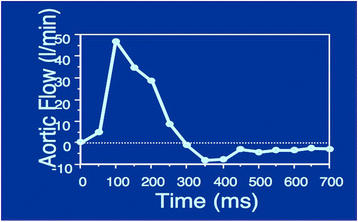
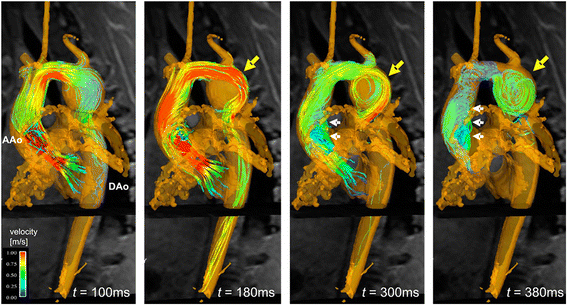
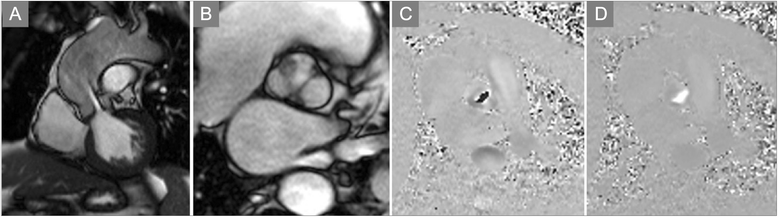
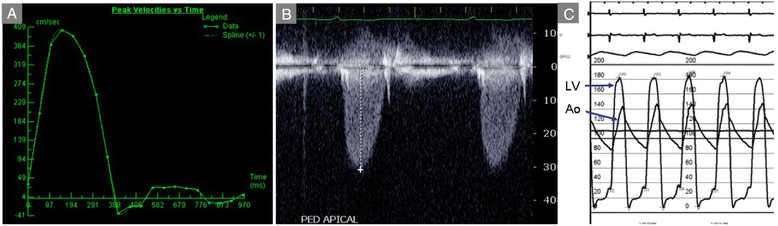
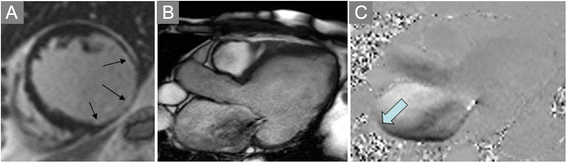
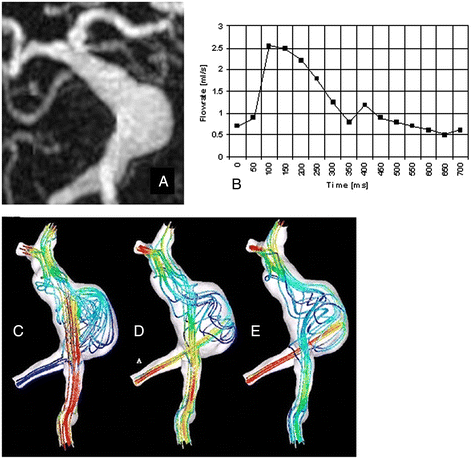
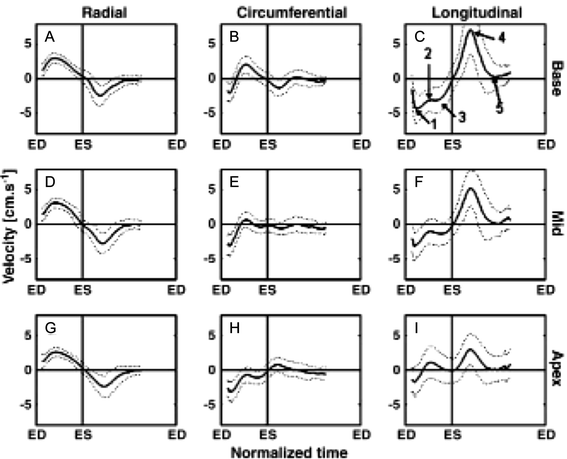
References
-
- Hahn EL. Detection of Sea-Water Motion by Nuclear precession. J Geophys Res. 1965;65:776–777.
-
- Singer JR. NMR diffusion and flow measurements and an introduction to spin phase graphing. J Phys E. 1978;11:281–291.
-
- Moran PR. A flow velocity zeugmatographic interlace for NMR imaging in humans. Magn Reson Imaging. 1982;1:197–203. - PubMed
-
- Bryant DJ, Payne JA, Firmin DN, Longmore DB. Measurement of flow with NMR imaging using a gradient pulse and phase difference technique. J Comput Assist Tomogr. 1984;8:588–593. - PubMed
-
- Van Dijk P. Direct cardiac NMR imaging of heart wall and blood flow velocity. J Comput Assist Tomogr. 1984;8:429–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

