Deficient vesicular storage: A common theme in catecholaminergic neurodegeneration
- PMID: 26255205
- PMCID: PMC4554767
- DOI: 10.1016/j.parkreldis.2015.07.009
Deficient vesicular storage: A common theme in catecholaminergic neurodegeneration
Abstract
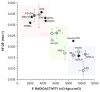
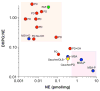
Several neurodegenerative diseases involve loss of catecholamine neurons--Parkinson's disease (PD) is a prototypical example. Catecholamine neurons are rare in the nervous system, and why they are lost has been mysterious. Accumulating evidence supports the concept of "autotoxicity"--inherent cytotoxicity caused by catecholamine metabolites. Since vesicular sequestration limits the buildup of toxic products of enzymatic and spontaneous oxidation of catecholamines, a vesicular storage defect could play a pathogenic role in the death of catecholaminergic neurons in a variety of neurodegenerative diseases. In putamen, deficient vesicular storage is revealed in vivo by accelerated loss of (18)F-DOPA-derived radioactivity and post-mortem by decreased tissue dopamine (DA):DOPA ratios; in myocardium in vivo by accelerated loss of (18)F-dopamine-derived radioactivity and post-mortem by increased 3,4-dihydroxyphenylglycol:norepinephrine (DHPG:NE) ratios; and in sympathetic noradrenergic nerves overall in vivo by increased plasma F-dihydroxyphenylacetic acid (F-DOPAC):DHPG ratios. We retrospectively analyzed data from 20 conditions with decreased or intact catecholaminergic innervation, involving different etiologies, pathogenetic mechanisms, and lesion locations. All conditions involving parkinsonism had accelerated loss of putamen (18)F-DOPA-derived radioactivity; in those with post-mortem data there were also decreased putamen DA:DOPA ratios. All conditions involving cardiac sympathetic denervation had accelerated loss of myocardial (18)F-dopamine-derived radioactivity; in those with post-mortem data there were increased myocardial DHPG:NE ratios. All conditions involving localized loss of catecholaminergic innervation had evidence of decreased vesicular storage specifically in the denervated regions. Thus, across neurodegenerative diseases, loss of catecholaminergic neurons seems to be associated with decreased vesicular storage in the residual neurons.
Keywords: Catecholamine; Dopamine; Neurodegeneration; Norepinephrine; Parkinson's disease.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest: The authors have no conflicts of interest to report.
Figures



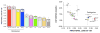



References
-
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. - PubMed
-
- Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs KA, Delledonne A, Parisi JE, Ahlskog JE, Dickson DW. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord. 2008;23:1085–1092. - PubMed
-
- Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. - PubMed
REFERENCES FOR APPENDIX
-
- Suzuki T, Higa S, Sakoda S, Hayashi A, Yamamura Y, Takaba Y, Nakajima A. Orthostatic hypotension in familial amyloid polyneuropathy: treatment with DL-threo-3,4-dihydroxyphenylserine. Neurology. 1981;31:1323–1326. - PubMed
-
- Nakata T, Shimamoto K, Yonekura S, Kobayashi N, Sugiyama T, Imai K, Iimura O. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: detection with iodine-123-MIBG. J Nucl Med. 1995;36:1040–1042. - PubMed
-
- Hofer PA, Anderson R. Postmortem findings in primary familial amyloidosis with polyneuropathy. Acta Pathol Microbiol Scand A. 1975;83:309–322. - PubMed
-
- Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res. 2002;12:281–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

