Interleukin-6 inhibits apoptosis of exocrine gland tissues under inflammatory conditions
- PMID: 26255211
- PMCID: PMC4605873
- DOI: 10.1016/j.cyto.2015.07.027
Interleukin-6 inhibits apoptosis of exocrine gland tissues under inflammatory conditions
Abstract
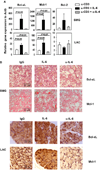
Interleukin (IL)-6 is a multi-functional cytokine that can either promote or suppress tissue inflammation depending on the specific disease context. IL-6 is elevated in the exocrine glands and serum of patients with Sjögren's syndrome (SS), but the specific role of IL-6 in the pathogenesis of this disease has not been defined. In this study, we showed that IL-6 expression levels were increased with age in C56BL/6.NOD-Aec1Aec2 mice, a primary SS model, and higher than the control C57BL/6 mice. To assess the role of IL-6 during the immunological phase of SS development, a neutralizing anti-IL-6 antibody was administered into 16 week-old female C56BL/6.NOD-Aec1Aec2 mice, 3 times weekly for a consecutive 8 weeks. Neutralization of endogenous IL-6 throughout the immunological phase of SS development led to increased apoptosis, caspase-3 activation, leukocytic infiltration, and IFN-γ- and TNF-α production in the salivary gland. To further determine the effect of IL-6 on the apoptosis of exocrine gland cells, recombinant human IL-6 or the neutralizing anti-IL-6 antibody was injected into female C57BL/6 mice that received concurrent injection of anti-CD3 antibody to induce the apoptosis of exocrine gland tissues. Neutralization of IL-6 enhanced, whereas administration of IL-6 inhibited apoptosis and caspase-3 activation in salivary and lacrimal glands in this model. The apoptosis-suppressing effect of IL-6 was associated with up-regulation of Bcl-xL and Mcl-1 in both glands. Moreover, IL-6 treatment induced activation of STAT3 and up-regulated Bcl-xL and Mcl-1 gene expression in a human salivary gland epithelial cell line. In conclusion, IL-6 inhibits the apoptosis of exocrine gland tissues and exerts a tissue-protective effect under inflammatory conditions including SS. These findings suggest the possibility of using this property of IL-6 to preserve exocrine gland tissue integrity and function under autoimmune and inflammatory conditions.
Keywords: Apoptosis; Exocrine gland; Inflammation; Interleukin-6; Sjögren’s syndrome.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 22:83–89. - PubMed
-
- Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. - PubMed
-
- Lindroos J, Svensson L, Norsgaard H, Lovato P, Moller K, Hagedorn PH, et al. IL-23-mediated epidermal hyperplasia is dependent on IL-6. The Journal of investigative dermatology. 131:1110–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

