Diffusion MRI and its Role in Neuropsychology
- PMID: 26255305
- PMCID: PMC4807614
- DOI: 10.1007/s11065-015-9291-z
Diffusion MRI and its Role in Neuropsychology
Abstract
Diffusion Magnetic Resonance Imaging (dMRI) is a popular method used by neuroscientists to uncover unique information about the structural connections within the brain. dMRI is a non-invasive imaging methodology in which image contrast is based on the diffusion of water molecules in tissue. While applicable to many tissues in the body, this review focuses exclusively on the use of dMRI to examine white matter in the brain. In this review, we begin with a definition of diffusion and how diffusion is measured with MRI. Next we introduce the diffusion tensor model, the predominant model used in dMRI. We then describe acquisition issues related to acquisition parameters and scanner hardware and software. Sources of artifacts are then discussed, followed by a brief review of analysis approaches. We provide an overview of the limitations of the traditional diffusion tensor model, and highlight several more sophisticated non-tensor models that better describe the complex architecture of the brain's white matter. We then touch on reliability and validity issues of diffusion measurements. Finally, we describe examples of ways in which dMRI has been applied to studies of brain disorders and how identified alterations relate to symptomatology and cognition.
Figures




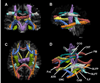
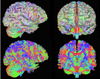
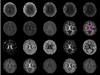
Similar articles
-
A survey of current trends in diffusion MRI for structural brain connectivity.J Neural Eng. 2016 Feb;13(1):011001. doi: 10.1088/1741-2560/13/1/011001. Epub 2015 Dec 22. J Neural Eng. 2016. PMID: 26695367 Review.
-
Potential and limitations of diffusion MRI tractography for the study of language.Brain Lang. 2014 Apr;131:65-73. doi: 10.1016/j.bandl.2013.06.007. Epub 2013 Jul 30. Brain Lang. 2014. PMID: 23910928 Review.
-
Tractometry of Human Visual White Matter Pathways in Health and Disease.Magn Reson Med Sci. 2024 Jul 1;23(3):316-340. doi: 10.2463/mrms.rev.2024-0007. Epub 2024 Jun 12. Magn Reson Med Sci. 2024. PMID: 38866532 Free PMC article. Review.
-
Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review.J Mol Neurosci. 2008;34(1):51-61. doi: 10.1007/s12031-007-0029-0. J Mol Neurosci. 2008. PMID: 18157658 Review.
-
Anatomically constrained tractography of the fetal brain.Neuroimage. 2024 Aug 15;297:120723. doi: 10.1016/j.neuroimage.2024.120723. Epub 2024 Jul 17. Neuroimage. 2024. PMID: 39029605 Free PMC article.
Cited by
-
Disruption of white matter integrity and its relationship with cognitive function in non-severe traumatic brain injury.Front Neurol. 2022 Oct 11;13:1011304. doi: 10.3389/fneur.2022.1011304. eCollection 2022. Front Neurol. 2022. PMID: 36303559 Free PMC article.
-
Relating white matter microstructure in theoretically defined addiction networks to relapse in alcohol use disorder.Cereb Cortex. 2023 Aug 23;33(17):9756-9763. doi: 10.1093/cercor/bhad241. Cereb Cortex. 2023. PMID: 37415080 Free PMC article.
-
Relationship Between Diffusion Tensor Imaging (DTI) Findings and Cognition Following Pediatric TBI: A Meta-Analytic Review.Dev Neuropsychol. 2016 Apr;41(3):176-200. doi: 10.1080/87565641.2016.1186167. Epub 2016 May 27. Dev Neuropsychol. 2016. PMID: 27232263 Free PMC article. Review.
-
The integrity of the corticospinal tract and corpus callosum, and the risk of ALS: univariable and multivariable Mendelian randomization.Sci Rep. 2024 Jul 26;14(1):17216. doi: 10.1038/s41598-024-68374-y. Sci Rep. 2024. PMID: 39060317 Free PMC article.
-
Causal Relationship between Aging and Anorexia Nervosa: A White-Matter-Microstructure-Mediated Mendelian Randomization Analysis.Biomedicines. 2024 Aug 16;12(8):1874. doi: 10.3390/biomedicines12081874. Biomedicines. 2024. PMID: 39200338 Free PMC article.
References
-
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain research. 1992;598(1–2):143–153. - PubMed
-
- Alexander DC. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;60(2):439–448. - PubMed
-
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage. 2005;27(1):48–58. - PubMed
-
- Assaf Y, Freidlin RZ, Rohde GK, Basser PJ. New modeling and experimental framework to characterize hindered and restricted water diffusion in brain white matter. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52(5):965–978. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

