Atypical antidepressants extend lifespan of Caenorhabditis elegans by activation of a non-cell-autonomous stress response
- PMID: 26255886
- PMCID: PMC4693466
- DOI: 10.1111/acel.12379
Atypical antidepressants extend lifespan of Caenorhabditis elegans by activation of a non-cell-autonomous stress response
Abstract
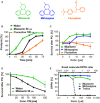
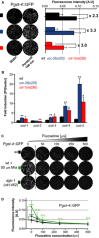
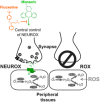
Oxidative stress has long been associated with aging and has recently been linked to psychiatric disorders, including psychosis and depression. We identified multiple antipsychotics and antidepressants that extend Caenorhabditis elegans lifespan and protect the animal from oxidative stress. Here, we report that atypical antidepressants activate a neuronal mechanism that regulates the response to oxidative stress throughout the animal. While the activation of the oxidative stress response by atypical antidepressants depends on synaptic transmission, the activation by reactive oxygen species does not. Lifespan extension by atypical antidepressants depends on the neuronal oxidative stress response activation mechanism. Neuronal regulation of the oxidative stress response is likely to have evolved as a survival mechanism to protect the organism from oxidative stress, upon detection of adverse or dangerous conditions by the nervous system.
Keywords: Caenorhabditis elegans; anti-aging; antidepressant; non-cell-autonomous; psychiatric disease; signal transduction; stress; synaptic transmission.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
-
- Abdel‐Wahab BA, Salama RH (2011) Venlafaxine protects against stress‐induced oxidative DNA damage in hippocampus during antidepressant testing in mice. Pharmacol. Biochem. Behav. 100, 59–65. - PubMed
-
- Barclay JW, Morgan A, Burgoyne RD (2012) Neurotransmitter release mechanisms studied in Caenorhabditis elegans . Cell Calcium 52, 289–295. - PubMed
-
- Bargmann CI (2012) Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34, 458–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

