Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration
- PMID: 26256209
- PMCID: PMC4550513
- DOI: 10.1016/j.devcel.2015.06.017
Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration
Abstract
Some organisms, such as adult zebrafish and newborn mice, have the capacity to regenerate heart tissue following injury. Unraveling the mechanisms of heart regeneration is fundamental to understanding why regeneration fails in adult humans. Numerous studies have revealed that nerves are crucial for organ regeneration, thus we aimed to determine whether nerves guide heart regeneration. Here, we show using transgenic zebrafish that inhibition of cardiac innervation leads to reduction of myocyte proliferation following injury. Specifically, pharmacological inhibition of cholinergic nerve function reduces cardiomyocyte proliferation in the injured hearts of both zebrafish and neonatal mice. Direct mechanical denervation impairs heart regeneration in neonatal mice, which was rescued by the administration of neuregulin 1 (NRG1) and nerve growth factor (NGF) recombinant proteins. Transcriptional analysis of mechanically denervated hearts revealed a blunted inflammatory and immune response following injury. These findings demonstrate that nerve function is required for both zebrafish and mouse heart regeneration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
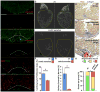



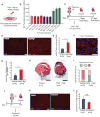


References
-
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. - PubMed
-
- Chen LN, Zang WJ, Yu XJ, Liu J, Li DL, Kong SS, Lu J, Xu XL. Compensatory recovery of vagal control of hemodynamics after unilateral vagotomy. Physiological research/Academia Scientiarum Bohemoslovaca. 2008;57:119–132. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

