Enantioselective Synthesis of Carbo- and Heterocycles through a CuH-Catalyzed Hydroalkylation Approach
- PMID: 26256576
- PMCID: PMC4558994
- DOI: 10.1021/jacs.5b07061
Enantioselective Synthesis of Carbo- and Heterocycles through a CuH-Catalyzed Hydroalkylation Approach
Abstract
The enantioselective, intramolecular hydroalkylation of halide-tethered styrenes has been achieved through a copper hydride-catalyzed process. This approach allowed for the synthesis of enantioenriched cyclobutanes, cyclopentanes, indanes, and six-membered N- and O-heterocycles. This protocol was applied to the synthesis of the commercial serotonin reuptake inhibitor (-)-paroxetine.
Figures
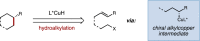
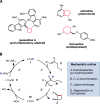

References
-
-
For selected reviews on the enantioselective catalytic formation of C(sp3)–C(sp3) bonds, see:
- Hoveyda A. H.; Morken J. P. Angew. Chem., Int. Ed. Engl. 1996, 35, 1262.10.1002/anie.199612621. - DOI
- Kobayashi S.; Ishitani H. Chem. Rev. 1999, 99, 1069.10.1021/cr980414z. - DOI - PubMed
- Fürstner A. Chem. Rev. 1999, 99, 991.10.1021/cr9703360. - DOI - PubMed
- Johnson J. S.; Evans D. A. Acc. Chem. Res. 2000, 33, 325.10.1021/ar960062n. - DOI - PubMed
- Mikami K.; Terada M.; Matsuzawa H. Angew. Chem., Int. Ed. 2002, 41, 3554.10.1002/1521-3773(20021004)41:19<3554::AID-ANIE3554>3.0.CO;2-P. - DOI - PubMed
- Davies H. M. L.; Beckwith R. E. J. Chem. Rev. 2003, 103, 2861.10.1021/cr0200217. - DOI - PubMed
- Ooi T.; Maruoka K. Angew. Chem., Int. Ed. 2007, 46, 4222.10.1002/anie.200601737. - DOI - PubMed
- Gaunt M. J.; Johansson C. C. C.; McNally A.; Vo N. T. Drug Discovery Today 2007, 12, 8.10.1016/j.drudis.2006.11.004. - DOI - PubMed
- Shibasaki M.; Kanai M. Chem. Rev. 2008, 108, 2853.10.1021/cr078340r. - DOI - PubMed
- Trost B. M.; Brindle C. S. Chem. Soc. Rev. 2010, 39, 1600.10.1039/b923537j. - DOI - PMC - PubMed
- Terada M. Bull. Chem. Soc. Jpn. 2010, 83, 101.10.1246/bcsj.20090268. - DOI
- Hassan A.; Krische M. J. Org. Process Res. Dev. 2011, 15, 1236.10.1021/op200195m. - DOI - PMC - PubMed
- Negishi E.-i. ARKIVOC 2010, 2011viii34.10.3998/ark.5550190.0012.803. - DOI - PubMed
- Watson I. D. G.; Toste F. D. Chem. Sci. 2012, 3, 2899.10.1039/c2sc20542d. - DOI
- Yang L.; Huang H. Catal. Sci. Technol. 2012, 2, 1099.10.1039/c2cy20111a. - DOI
- Scheffler U.; Mahrwald R. Chem. - Eur. J. 2013, 19, 14346.10.1002/chem.201301996. - DOI - PubMed
- Tsubogo T.; Ishiwata T.; Kobayashi S. Angew. Chem., Int. Ed. 2013, 52, 6590.10.1002/anie.201210066. - DOI - PubMed
- Šebesta R. ChemCatChem 2013, 5, 1069.10.1002/cctc.201200926. - DOI
- Tasker S. Z.; Standley E. A.; Jamison T. F. Nature 2014, 509, 299.10.1038/nature13274. - DOI - PMC - PubMed
-
For selected methods based on stoichiometric auxiliaries, see:
- Meyers A. I. Acc. Chem. Res. 1978, 11, 375.10.1021/ar50130a002. - DOI
- Evans D. A.; Bartroli J.; Shih T. L. J. Am. Chem. Soc. 1981, 103, 2127.10.1021/ja00398a058. - DOI
- Myers A. G.; Yang B. H.; Chen H.; McKinstry L.; Kopecky D. J.; Gleason J. L. J. Am. Chem. Soc. 1997, 119, 6496.10.1021/ja970402f. - DOI
- Ellman J. A.; Owens T. D.; Tang T. P. Acc. Chem. Res. 2002, 35, 984.10.1021/ar020066u. - DOI - PubMed
- Job A.; Janeck C. F.; Bettray W.; Peters R.; Enders D. Tetrahedron 2002, 58, 2253.10.1016/S0040-4020(02)00080-7. - DOI
-
For an overview of enantioselective C—C bond-forming reactions applied to complex molecule synthesis, see:
- Corey E. J.; Kürti L.. Enantioselective Chemical Synthesis; Academic Press: San Diego, 2013.
-
-
-
For examples of the enantioselective, catalytic generation of C(sp3)–C(sp3) bonds remote from reactive functional groups, see:
- Pino P.; Cioni P.; Wei J. J. Am. Chem. Soc. 1987, 109, 6189.10.1021/ja00254a052. - DOI
- Long J.; Du H.; Li K.; Shi Y. Tetrahedron Lett. 2005, 46, 2737.10.1016/j.tetlet.2005.02.161. - DOI
- Arp F. O.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 10482.10.1021/ja053751f. - DOI - PubMed
- Saito B.; Fu G. C. J. Am. Chem. Soc. 2008, 130, 6694.10.1021/ja8013677. - DOI - PubMed
-
-
- Corey E. J.; Posner G. H. J. Am. Chem. Soc. 1967, 89, 3911.10.1021/ja00991a049. - DOI
- Whitesides G. M.; Fischer W. P.; San Filippo J.; Bashe R. W.; House H. O. J. Am. Chem. Soc. 1969, 91, 4871.10.1021/ja01045a049. - DOI
-
For the copper-catalyzed coupling of alkyl halides and Grignard reagents, see:
- Fouquet G.; Schlosser M. Angew. Chem., Int. Ed. Engl. 1974, 13, 82.10.1002/anie.197400821. - DOI
-
-
For the construction of C(sp3)–C(sp3) bonds through Cu-catalyzed alkene carboboration, see:
- Ito H.; Kosaka Y.; Nonoyama K.; Sasaki Y.; Sawamura M. Angew. Chem., Int. Ed. 2008, 47, 7424.10.1002/anie.200802342. - DOI - PubMed
- Ito H.; Toyoda T.; Sawamura M. J. Am. Chem. Soc. 2010, 132, 5990.10.1021/ja101793a. - DOI - PubMed
- Zhong C.; Kunii S.; Kosaka Y.; Sawamura M.; Ito H. J. Am. Chem. Soc. 2010, 132, 11440.10.1021/ja103783p. - DOI - PubMed
- Kubota K.; Yamamoto E.; Ito H. J. Am. Chem. Soc. 2013, 135, 2635.10.1021/ja3104582. - DOI - PubMed
- Yoshida H.; Kageyuki I.; Takaki K. Org. Lett. 2013, 15, 952.10.1021/ol4001526. - DOI - PubMed
-
-
- Zhu S.; Niljianskul N.; Buchwald S. L. J. Am. Chem. Soc. 2013, 135, 15746.10.1021/ja4092819. - DOI - PMC - PubMed
- Zhu S.; Buchwald S. L. J. Am. Chem. Soc. 2014, 136, 15913.10.1021/ja509786v. - DOI - PMC - PubMed
- Niljianskul N.; Zhu S.; Buchwald S. L. Angew. Chem., Int. Ed. 2015, 54, 1638.10.1002/anie.201410326. - DOI - PMC - PubMed
- Shi S.; Buchwald S. L. Nat. Chem. 2014, 7, 38.10.1038/nchem.2131. - DOI - PMC - PubMed
-
For the independent development of a related system, see:
- Miki Y.; Hirano K.; Satoh T.; Miura M. Angew. Chem., Int. Ed. 2013, 52, 10830.10.1002/anie.201304365. - DOI - PubMed
- Miki Y.; Hirano K.; Satoh T.; Miura M. Org. Lett. 2014, 16, 1498.10.1021/ol5003219. - DOI - PubMed
-
For a CuH-catalyzed C—C bond-forming reaction resulting in the enantioselective formation of indolines, see:
- Ascic E.; Buchwald S. L. J. Am. Chem. Soc. 2015, 137, 4666.10.1021/jacs.5b02316. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

