MBNL Sequestration by Toxic RNAs and RNA Misprocessing in the Myotonic Dystrophy Brain
- PMID: 26257173
- PMCID: PMC4545389
- DOI: 10.1016/j.celrep.2015.07.029
MBNL Sequestration by Toxic RNAs and RNA Misprocessing in the Myotonic Dystrophy Brain
Abstract
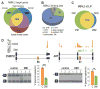
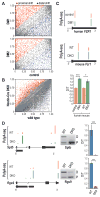
For some neurological disorders, disease is primarily RNA mediated due to expression of non-coding microsatellite expansion RNAs (RNA(exp)). Toxicity is thought to result from enhanced binding of proteins to these expansions and depletion from their normal cellular targets. However, experimental evidence for this sequestration model is lacking. Here, we use HITS-CLIP and pre-mRNA processing analysis of human control versus myotonic dystrophy (DM) brains to provide compelling evidence for this RNA toxicity model. MBNL2 binds directly to DM repeat expansions in the brain, resulting in depletion from its normal RNA targets with downstream effects on alternative splicing and polyadenylation. Similar RNA processing defects were detected in Mbnl compound-knockout mice, highlighted by dysregulation of Mapt splicing and fetal tau isoform expression in adults. These results demonstrate that MBNL proteins are directly sequestered by RNA(exp) in the DM brain and introduce a powerful experimental tool to evaluate RNA-mediated toxicity in other expansion diseases.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Carpentier C, Ghanem D, Fernandez-Gomez FJ, Jumeau F, Philippe JV, Freyermuth F, Labudeck A, Eddarkaoui S, Dhaenens CM, Holt I, et al. Tau exon 2 responsive elements deregulated in myotonic dystrophy type I are proximal to exon 2 and synergistically regulated by MBNL1 and MBNL2. Biochim Biophys Acta. 2014;1842:654–664. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

