TPX2 regulates neuronal morphology through kinesin-5 interaction
- PMID: 26257190
- PMCID: PMC4564317
- DOI: 10.1002/cm.21234
TPX2 regulates neuronal morphology through kinesin-5 interaction
Abstract
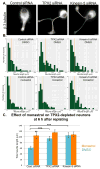
TPX2 (targeting protein for Xklp2) is a multifunctional mitotic spindle assembly factor that in mammalian cells localizes and regulates mitotic motor protein kinesin-5 (also called Eg5 or kif11). We previously showed that upon depletion or inhibition of kinesin-5 in cultured neurons, microtubule movements increase, resulting in faster growing axons and thinner dendrites. Here, we show that depletion of TPX2 from cultured neurons speeds their rate of process outgrowth, similarly to kinesin-5 inhibition. The phenotype is rescued by TPX2 re-expression, but not if TPX2's kinesin-5-interacting domain is deleted. These results, together with studies showing a spike in TPX2 expression during dendritic differentiation, suggest that the levels and distribution of TPX2 are likely to be determinants of when and where kinesin-5 acts in neurons.
Keywords: Eg5; TPX2; kif11; microtubule; molecular motor; neuron.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Authors declare no conflict of interest
Figures


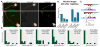
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

